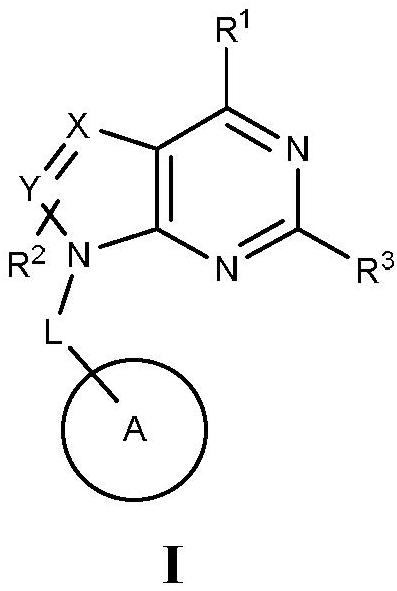
Pyrimido five-membered heterocyclic compounds or pharmaceutically acceptable salts and isomers thereof, preparation method of pyrimido five-membered heterocyclic compounds or pharmaceutically acceptable salts and isomers thereof, pharmaceutical compositions and application of pyrimido five-membered heterocyclic compounds or pharmaceutically acceptable salts and isomers thereof and pharmaceutical compositions
A five-membered heterocycle and compound technology, applied in the field of chemistry and medicine, can solve problems such as poor membrane permeability and metabolic instability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0525] Example 1: Synthesis of Compound S1
[0526] 2-(2-Amino-6-(propylamino)-9H-purin-9-yl)-N-(1-ethyl-3-methyl-1H-pyrazol-5-yl)carboxamide (S1 )
[0527]
[0528] Step 1: Synthesis of Compound 2
[0529] Raw material 1 (6.8 g, 40 mmol) was weighed and dissolved in anhydrous tetrahydrofuran, Boc anhydride (38 mL, 160 mmol) was added at 0° C., DMAP (492 mg, 4 mmol) was added, and the reaction was carried out overnight at room temperature. The next day, spin off tetrahydrofuran under reduced pressure, add EA (300mL) to redissolve, wash with 1M hydrochloric acid solution (25mL×2), wash with water (30mL) once, wash once with saturated brine (30mL), dry with anhydrous sodium sulfate, filter, The solvent was evaporated to dryness to give a yellow oil. The yellow oil was dissolved in methanol (300 mL) and saturated NaHCO was added 3 The solution (200 mL) was heated to 50° C. for 1 h, transferred to room temperature, and reacted overnight. The next day, after the reaction wa...
Embodiment 2
[0540] Example 2: Synthesis of Compound S132
[0541] N-(2-(2-Amino-6-(pyrrol-1-yl)-9H-purin-9-yl)ethyl)-1-ethyl-3-methyl-1H-pyrazole-5-methyl Amide (S132)
[0542]
[0543] Step 1: Synthesis of Compound 7
[0544] Compound 2 (200 mg, 0.54 mmol) was weighed and dissolved in 1,4-dioxane, tetrahydropyrrole (44 mg, 0.60 mmol) and TEA (0.15 mL, 1.08 mmol) were added, and the temperature was raised to 60 °C and stirred for 3 h. . Stop the reaction, spin off 1,4-dioxane, add 10 mL of DCM to dissolve, wash with water, wash with saturated brine, dry over anhydrous sodium sulfate, filter, evaporate the solvent to dryness, make sand, and purify by column chromatography (DCM:MeOH=60 : 1 to 30: 1) to obtain compound 7 (163 mg, 75%). 1 H NMR (300 MHz, Chloroform-d) δ: 7.99 (d, J=1.8 Hz, 1H), 3.84-3.77 (m, 2H), 2.09-2.01 (m, 2H), 1.48 (s, 9H).
[0545] Step 2: Synthesis of Compound 8
[0546] Weigh compound 7 (163 mg, 0.4 mmol) and dissolve it in anhydrous THF (5 mL), add N-CBZ-eth...
Embodiment 3
[0553] Example 3: Synthesis of Compound 147
[0554] N-(2-(2-Amino-6-(cyclopropylamino)-9H-purin-9-yl)ethyl)-1-ethyl-3-methyl-1H-pyrazole-5-carboxamide ( S147)
[0555]
[0556] Step 1: Synthesis of Compound 11
[0557] Compound 2 (200 mg, 0.54 mmol) was weighed and dissolved in 1,4-dioxane, cyclopropylamine (34.2 mg, 0.60 mmol) and TEA (0.15 mL, 1.08 mmol) were added, the temperature was raised to 60 °C and the reaction was stirred for 3 h . Stop the reaction, spin off 1,4-dioxane, add 10 mL of DCM to dissolve, wash with water, wash with saturated brine, dry over anhydrous sodium sulfate, filter, evaporate the solvent to dryness, make sand, and purify by column chromatography (DCM:MeOH=60 : 1 to 30: 1) to obtain compound 10 (166 mg, 79%). 1 H NMR (300 MHz, Chloroform-d) δ: 7.98 (d, J=1.8 Hz, 1H), 6.19 (d, J=9.5 Hz, 1H), 3.03 (m, 1H), 1.48 (s, 18H), 0.70 -0.55(m, 4H).
[0558] Step 2: Synthesis of Compound 12
[0559] Compound 11 (156 mg, 0.40 mmol) was weighed and d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com