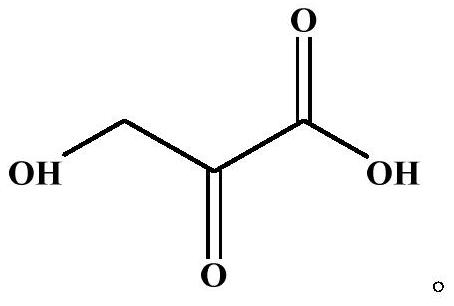
Application of beta-hydroxypyruvic acid in preparation of human islet amyloid polypeptide aggregation inhibitor
A hydroxypyruvate and amyloid polypeptide technology, which can be used in the fields of anhydride/acid/halide active ingredients, metabolic diseases, drug combinations, etc., and can solve the problems of restriction, low bioavailability and low gastrointestinal absorption rate.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
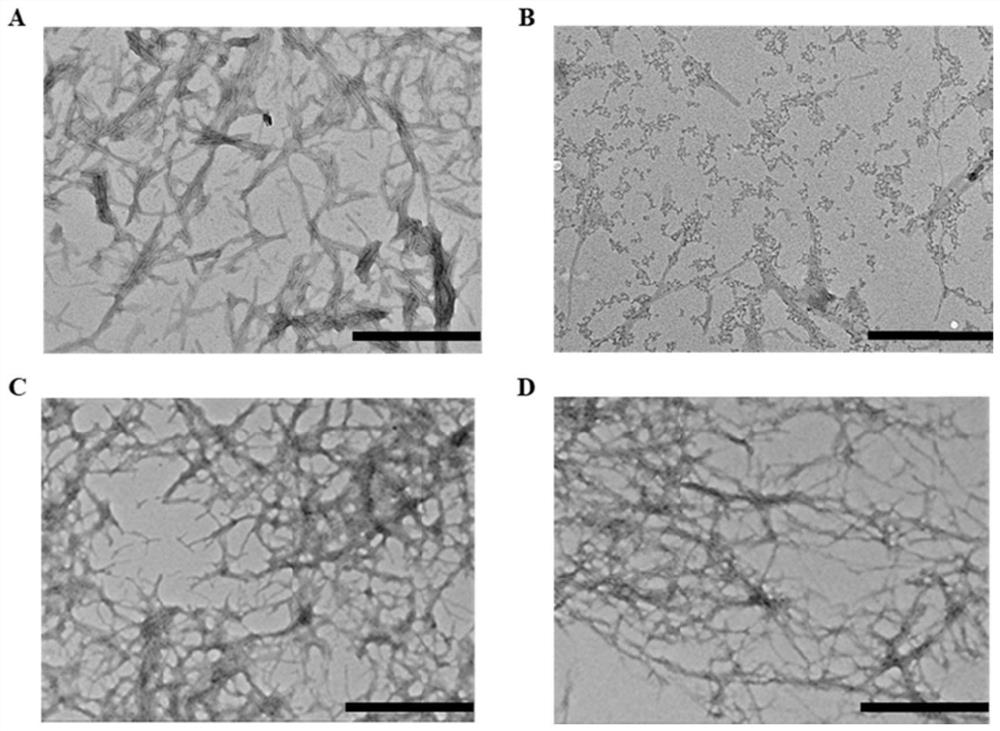
[0022] In this example, transmission electron microscopy is used to characterize the effect of candidate metabolites on the morphology of fibers formed by IAPP aggregation.
[0023] 1. IAPP dispersion method: Weigh 4.74mg IAPP, measure 2.456mL HFIP to dissolve, the concentration is 0.5mM, ultrasonic for 2 minutes.
[0024] 2. Prepare 2.5×PBS (containing 50mM NaCl) solution: weigh 0.2g KCl, 1.1688g NaCl, 0.22gKH 2 PO 4 ,2.08g Na 2 HPO 4 12H 2 O, measure 250mL ultrapure water to dissolve it completely.
[0025] 3. Preparation of HPA stock solution: Weigh 5 mg of HPA, add 600 μL of the above-mentioned 2.5×PBS (containing 50 mM NaCl) solution, dissolve it, and prepare 80 mM HPA stock solution.
[0026] 4. Prepare 3-methyl-L-histidine stock solution: weigh 1mg of 3-methyl-L-histidine, add 739μL of the above-mentioned 2.5×PBS (containing 50mM NaCl) solution, dissolve it, and prepare Make 8mM 3-methyl-L-histidine stock solution.
[0027] 5. Preparation of 3-hydroxybutyric acid...
Embodiment 2
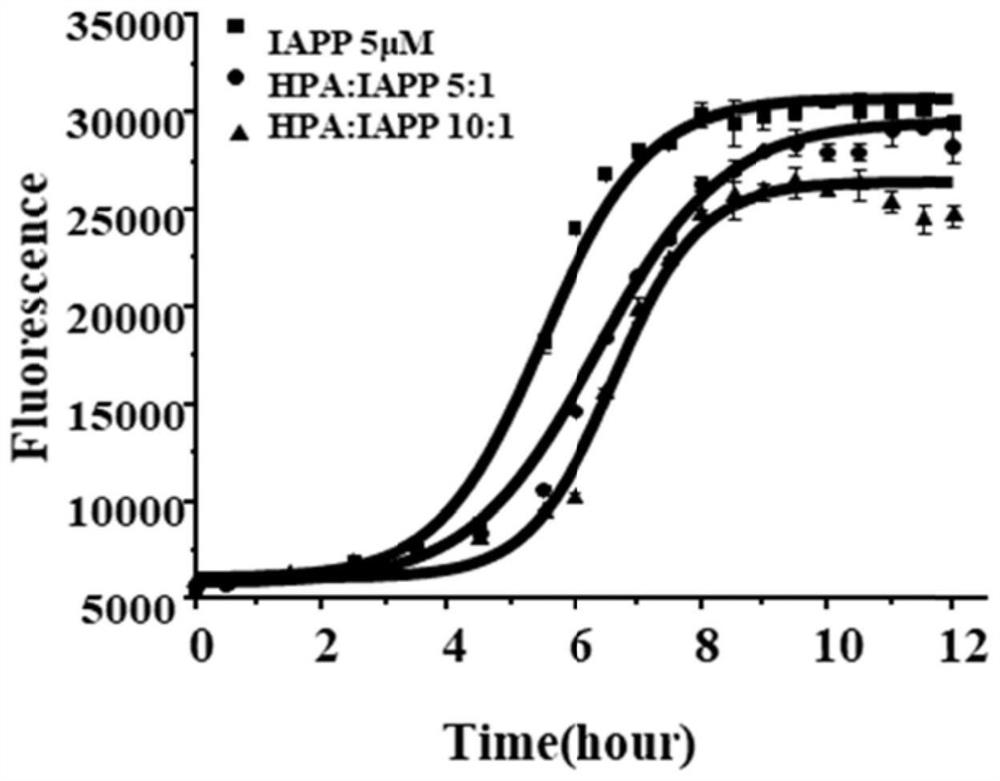
[0035] In this example, ThT staining was used to characterize the regulation of HPA on the aggregation ability of IAPP by fluorescence spectrophotometry.
[0036] 1. Prepare 2.5×PBS (containing 50mM NaCl) solution: weigh 0.2g KCl, 1.1688g NaCl, 0.22gKH 2 PO 4 ,2.08g Na 2 HPO 4 12H 2O, measure 250mL ultrapure water to dissolve it completely.
[0037] 2. Prepare ThT stock solution: Weigh 1.25mg ThT and dissolve it in 1.960mL ultrapure water.
[0038] 3. HPA stock solution method: as described in Example 1.
[0039] 4. IAPP dispersion method: as described in Example 1.
[0040] 5. Measure 500 μL of the above-prepared dispersed IAPP stock solution and add it to 49.5 mL of the above-mentioned 2.5×PBS (containing 50 mM NaCl) to prepare a control group.
[0041] 6. Take 16 μL of HPA stock solution, add it to 49.484 mL of 2.5×PBS (50 mM NaCl), mix with 500 μL of the dispersed IAPP stock solution prepared above, and prepare HPA:IAPP 5:1 group.
[0042] 7. Take 32 μL of HPA stoc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com