2-phosphonyl-3-fluoro vinyl indole compound and preparation method thereof
A technology of fluorovinyl indole compound and difluoromethyl indole carbinol, which is applied in the field of 2-phosphono-3-fluorovinyl indole compound and preparation thereof, can solve harsh anhydrous and anoxic conditions, Difficulty in synthesizing raw materials, expensive photosensitizers, etc., to achieve a wide range of applicable substrates, green reaction solvents, and good compatibility.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] The reaction formula is:
[0038]
[0039] A kind of synthetic method of 2-phosphono-3-fluorovinylindole compound: get 25mL round bottom flask, add difluoromethyl indole carbinol 2a (0.4mmol), diphenoxyphos 3a (0.6mmol), 95% ethanol (1 ml), stirred and reacted at 80°C for 6h, after the reaction was completed, cooled to room temperature, concentrated the reaction solution by rotary evaporation, and separated by silica gel column chromatography (eluent: ethyl acetate:petroleum ether=40: 60), the target product 1aa (161.0 mg, white solid, yield 92%) was obtained.
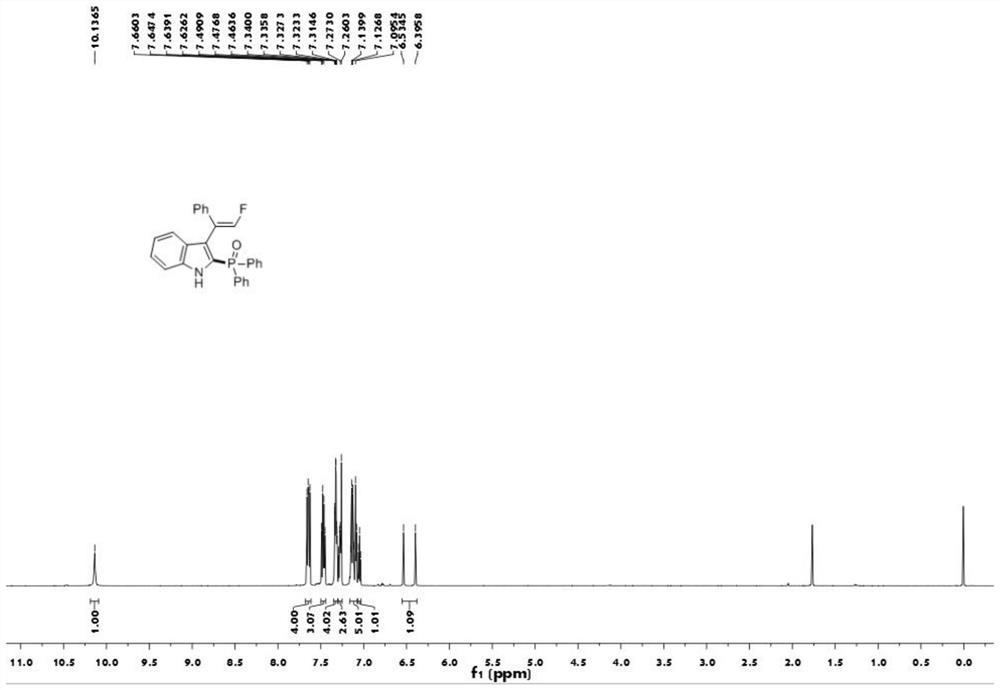
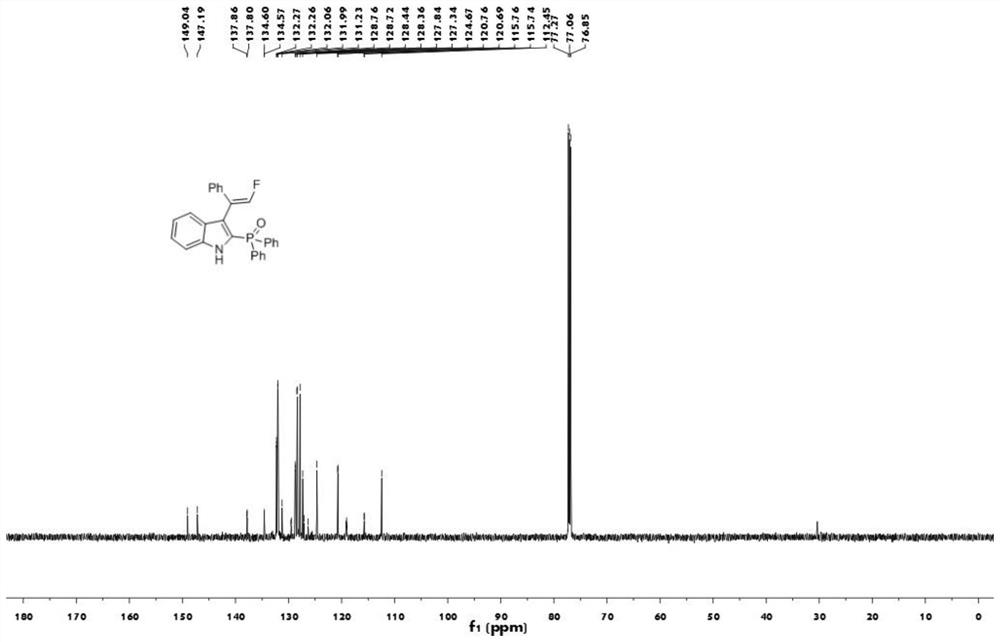
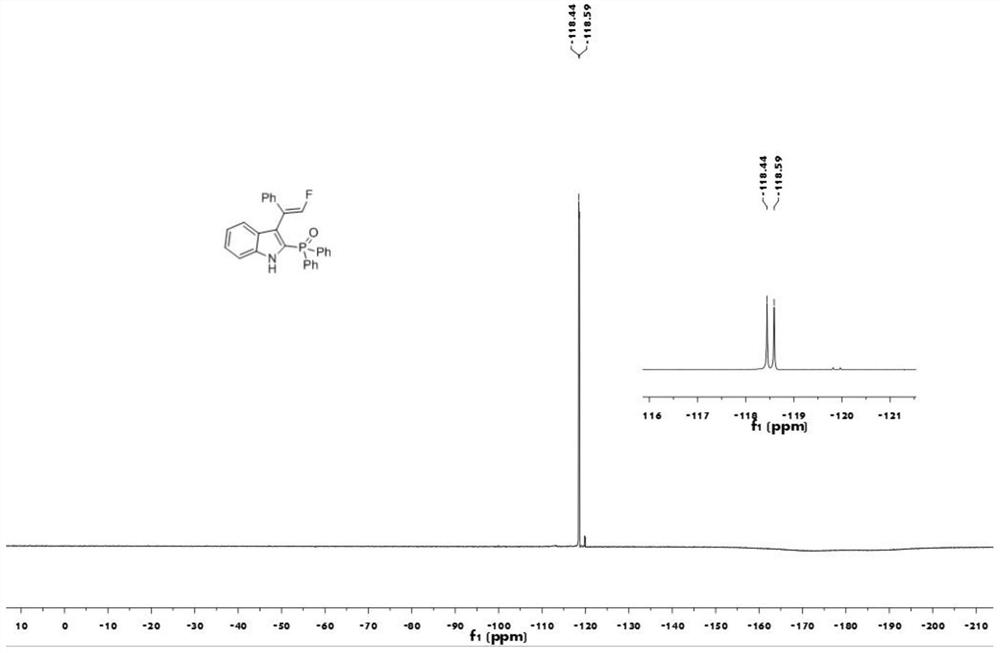
[0040] The proton nuclear magnetic resonance spectrum figure of the compound 1aa prepared in embodiment 1 is as follows figure 1 Shown; The carbon nuclear magnetic resonance spectrogram of the compound 1aa prepared in embodiment 1 is as figure 2 Shown; The nuclear magnetic resonance fluorine spectrogram of the compound 1aa prepared in embodiment 1 is as image 3 Shown; The nuclear magnetic resonance phosp...
Embodiment 2
[0043] The reaction formula is:
[0044]
[0045] A kind of synthetic method of 2-phosphono-3-fluorovinylindole compound: get 25mL round bottom flask, add difluoromethyl indole carbinol 2b (0.4mmol), diphenoxyphos 3a (0.6mmol), 95% ethanol (1 ml), stirred and reacted at 80°C for 24h, after the reaction was completed, cooled to room temperature, concentrated the reaction solution by rotary evaporation, and separated by silica gel column chromatography (eluent: ethyl acetate:petroleum ether=50: 50), the target product 1ba (161.4 mg, white solid, yield 89%) was obtained.
[0046] 1 H NMR (600MHz, DMSO-d 6 )δ11.15(s,1H),8.83(s,1H),7.70(dd,J=12.1,7.7Hz,4H),7.53(t,J=7.3Hz,2H),7.44(td,J=7.3 ,1.5Hz,4H),7.30(d,J=8.8Hz,1H),7.25-7.19(m,3H),7.14(d,J=7.0Hz,2H),6.91(t,J=84.1Hz,1H ),6.75(dd,J=8.8,1.7Hz,1H),6.29(d,J=1.3Hz,1H); 13 C NMR (150MHz, DMSO-d 6 )δ151.8, 148.0(d, J=275.5Hz), 134.8(d, J=2.0Hz), 133.2(d, J=10.1Hz), 133.1(d, J=106.8Hz), 132.4(d, J=2.2 Hz), 131.8(d, J=10.0Hz), 1...
Embodiment 3
[0048] The reaction formula is:
[0049]
[0050] A kind of synthetic method of 2-phosphono-3-fluorovinylindole compound: get 25mL round bottom flask, add difluoromethyl indole carbinol 2c (0.4mmol), diphenoxyphos 3a (0.6mmol), Ethanol (95%) (1 ml), stirred and reacted at 80°C for 12h, after the reaction was completed, cooled to room temperature, concentrated the reaction solution by rotary evaporation, and separated by silica gel column chromatography (eluent was ethyl acetate:petroleum ether= 50:50), the target product 1ca (168.5 mg, white solid, yield 85%) was obtained.
[0051] 1 H NMR (600MHz, CDCl 3 )δ10.75(s,1H),8.09(s,1H),7.97(dd,J=8.8,1.4Hz,1H),7.63-7.56(m,4H),7.50(d,J=8.7Hz,1H ),7.47(td,J=7.5,1.2Hz,2H),7.31(td,J=7.8,3.1Hz,4H),7.1-7.09(m,3H),7.05-7.01(m,2H),6.38( d,J=82.8Hz,1H),3.85(s,3H); 13 CNMR (150MHz, CDCl 3 )δ148.1(d, J=279.8Hz), 140.5(d, J=11.0Hz), 134.4(d, J=4.0Hz), 132.4, 132.0(d, J=10.8Hz), 131.0(d, J =112.0Hz), 129.2(d, J=12.1Hz), 128.5(d, J=7.1Hz...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com