Human anti-human tigit antibody and its application
An antibody and single-chain antibody technology, applied in the field of biomedicine, can solve the problems of limited benefited patients, poor tumor effect, and unclear reasons, and achieve the effect of enhancing killing activity, superior killing activity, and promoting killing activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0121] Embodiment 1, the generation of human source anti-human TIGIT antibody
[0122] 1. Materials
[0123] 1. Materials: The large-capacity fully synthetic phage single-chain antibody library was constructed by the Institute of Bioengineering of the former Academy of Military Medical Sciences (now the Academy of Military Medical Sciences) of the Chinese People's Liberation Army (ZL200910091261.8), with a library capacity of 1.35×10 10 . The host strain for phage infection was XL1-Blue (Stratagene, USA), the plasmid amplification strain was Top10 (Beijing Quanshijin Biotechnology Co., Ltd.), the helper phage was M13KO7 (Invitrogene, USA), horseradish catalase (HRP )-labeled anti-M13KO7 antibody (11973-MM05T-H) is a product of Beijing Sino Biological Technology Co., Ltd. CHO cells and TransIntro EL Transfection Reagent (L20313) were purchased from Beijing Quanshijin Biotechnology Co., Ltd. Opti-MEM serum-free medium for transfection and 1640 medium for cell culture, 0.25% t...
Embodiment 2
[0149] Example 2, the binding activity of AET2010
[0150] 1. Materials and methods
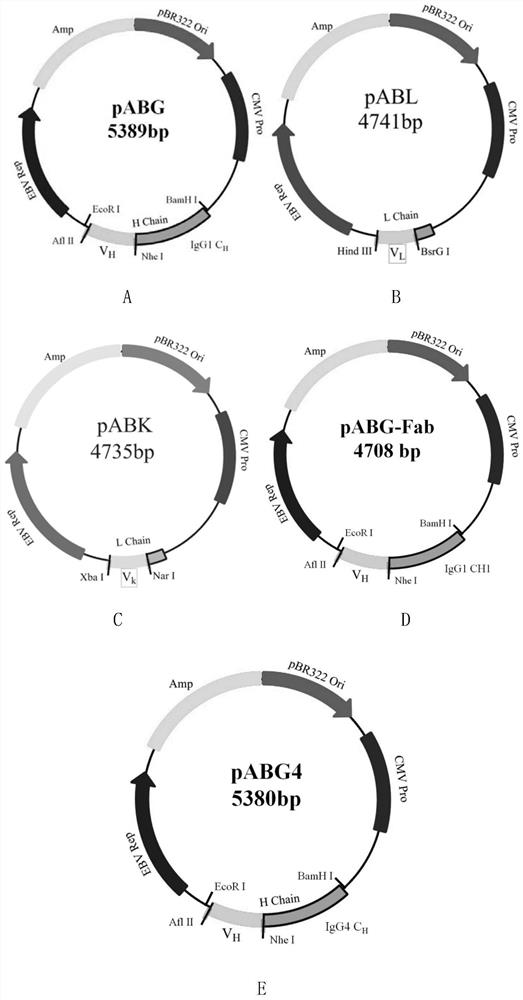
[0151] 1. Materials: Anti-TIGIT monoclonal antibody MK7684 can be obtained from Merck & Co., USA, and its light and heavy chain genes can also be synthesized and cloned into pABK ( figure 1 ) and pABG vectors were prepared according to the preparation method of AET2010 in Example 1 (patent number: US20160355589A1), the light chain and heavy chain amino acid sequences of MK7684 are shown in SEQ ID NO.11 and SEQ ID NO.12 respectively, and the light chain of MK7684 The gene sequences of the heavy chain and heavy chain are respectively shown in SEQ ID NO.13 and SEQ ID NO.14, and MK7684 is used as a control antibody.
[0152] Wherein, the light chain expression vector of MK7684 is a recombinant vector obtained by replacing the recognition sequence between Xba I and Nar I of the pABK expression vector with the light chain variable region gene shown in positions 1-327 of SEQ ID NO.13, The recombin...
Embodiment 3
[0178] Example 3, the blocking activity of AET2010
[0179] 1. Materials and methods
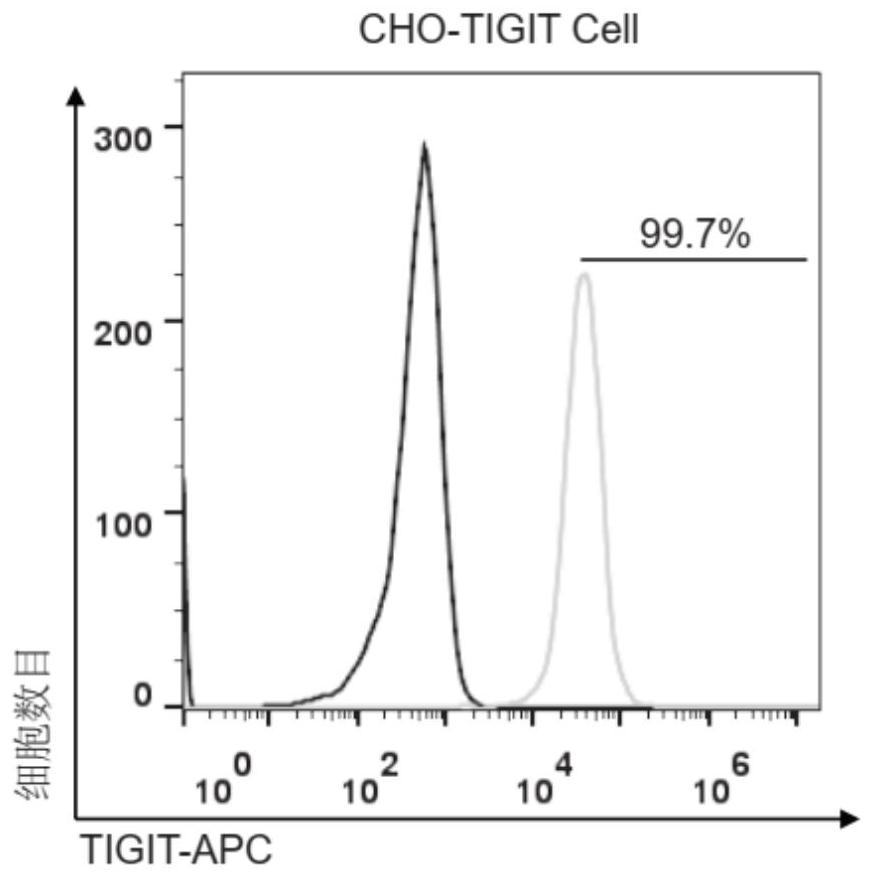
[0180] 1. Materials: The human CD155 (HG29682-UT) cDNA cloning vector is a product of Beijing Sino Biological Technology Co., Ltd. HiTrap for protein purification TM The Q-Sepharose FF anion adsorption column was produced by GE, the FITC-labeled mouse anti-human IgG / Fc flow antibody was produced by Biolegend, and the HRP-labeled goat anti-human IgG / Fc antibody was produced by Beijing Baixinyi Biotechnology Co., Ltd. The isotype control antibody (C103S) is a product of Beijing Sino Biological Technology Co., Ltd. Other related material sources are with embodiment 1,2.
[0181] 2. Method:
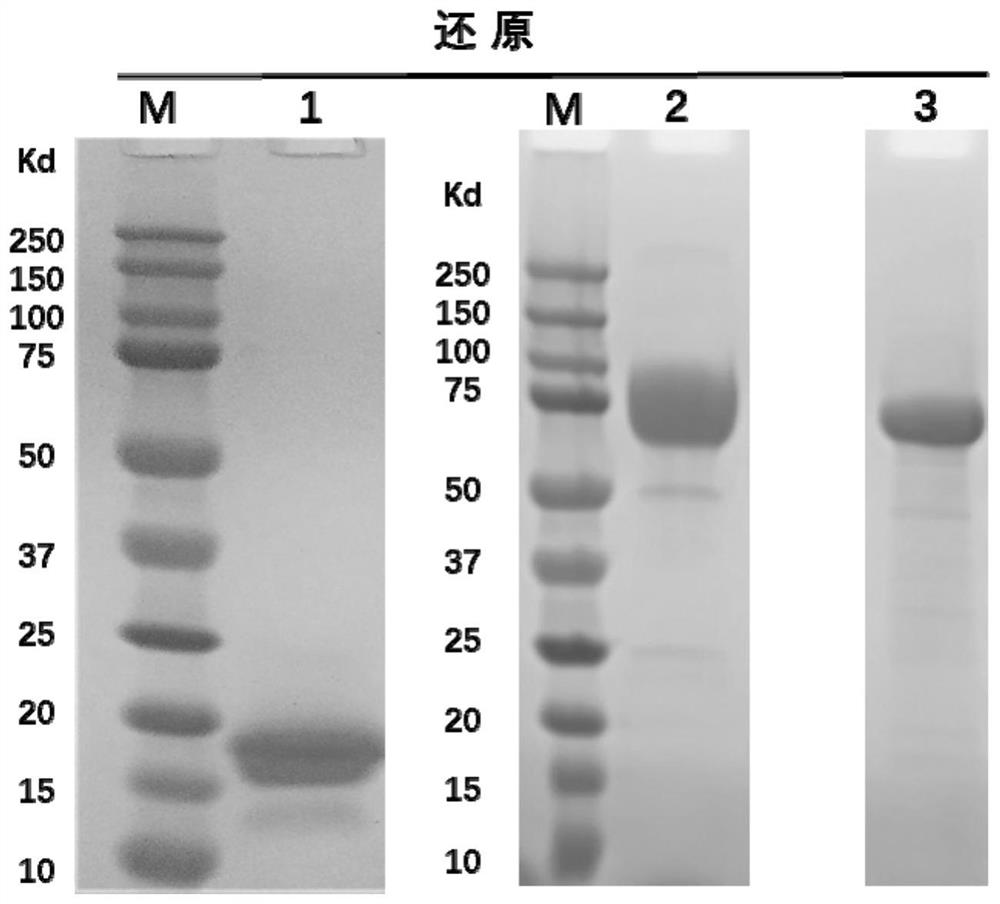
[0182] 2.1 Preparation of AET2010-Fab, MK7684-Fab and CD155-Fc recombinant protein
[0183] The antibody Fab segment heavy chain expression vector is a pABG vector without CH2 and CH3 gene sequences, and its name is pABG-Fab ( figure 1 , whose sequence is SEQ ID NO.22 in the sequence listing), the he...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com