Polymorphs of isopropylsulfonylphenylpyrimidines or salts thereof
A technology of polymorphs and compounds, applied in the field of medicine, can solve problems affecting dissolution, absorption, clinical efficacy and safety of drugs, and no research on polymorphs of compounds of formula I
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0173] 5-Chloro-N 2 -(5-Methyl-4-(piperidin-4-yl)-2-((propan-2-yl-d 7 )oxy)phenyl)-N 4 -(2-((Propan-2-yl-d 7 ) Preparation of polymorph I of sulfonyl)phenyl)pyrimidine-2,4-diamine
[0174] In the 200L reactor, 5-chloro-N was added successively 2 -(5-Methyl-4-(piperidin-4-yl)-2-((propan-2-yl-d 7 )oxy)phenyl)-N 4 -(2-((Propan-2-yl-d 7 )sulfonyl)phenyl)pyrimidine-2,4-diamine dihydrochloride (5.05 kg), acetone (21.0 kg) and purified water (7.2 kg). The temperature was raised to 55±5°C, and stirred at this temperature until the solid was dissolved. Purified water (15.0kg) and sodium hydroxide (0.93kg) were mixed, and the solution was stirred to prepare a sodium hydroxide solution. After the sodium hydroxide solution was filtered by pressure, it was added to the above kettle. After adding, keep stirring at 55±5°C, and then lower the temperature to 20±3°C. Purified water (38.0 kg) was slowly added, followed by stirring for 2±0.5 h. The obtained mixture was separated by cen...
Embodiment 2
[0183] 5-Chloro-N 2 -(5-Methyl-4-(piperidin-4-yl)-2-((propan-2-yl-d 7 )oxy)phenyl)-N 4 -(2-((Propan-2-yl-d 7 ) Preparation of polymorph I of sulfonyl)phenyl)pyrimidine-2,4-diamine
[0184] Take 7g of 5-chloro-N 2 -(5-Methyl-4-(piperidin-4-yl)-2-((propan-2-yl-d 7 )oxy)phenyl)-N 4 -(2-((Propan-2-yl-d 7 )sulfonyl)phenyl)pyrimidine-2,4-diamine dihydrochloride was added to the reaction flask, followed by adding 21 mL of a mixed solution (ethanol / water=3:1). The resulting mixture was heated to 55±3°C and stirred at this temperature until the solution was clear, then 25.4 g of aqueous sodium hydroxide solution (5.8% wt) was slowly added dropwise. After the addition, keep the temperature and continue to stir for 2 hours, then cool down to 20±3°C, then dropwise add water, and filter with suction after the addition. After the filter cake was washed with water three times, it was vacuum-dried to obtain a white solid, which weighed 5.6 g, and the yield was 90%. Its X-ray powder d...
Embodiment 3
[0186] 5-Chloro-N 2 -(5-Methyl-4-(piperidin-4-yl)-2-((propan-2-yl-d 7 )oxy)phenyl)-N 4 -(2-((Propan-2-yl-d 7 ) Preparation of polymorph I of sulfonyl)phenyl)pyrimidine-2,4-diamine
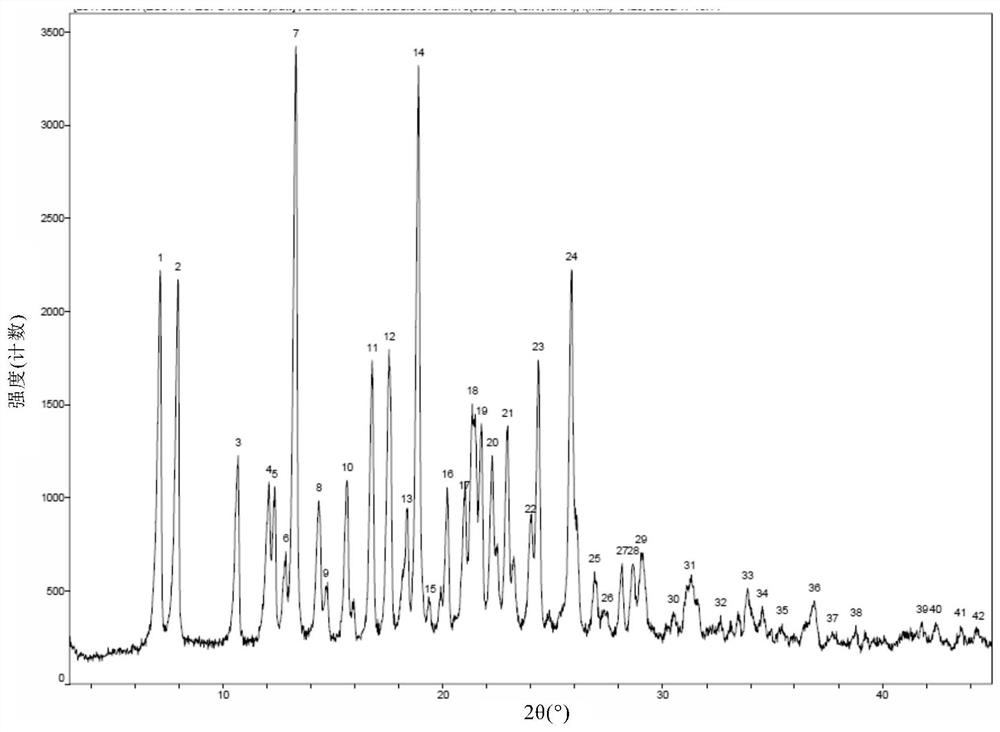
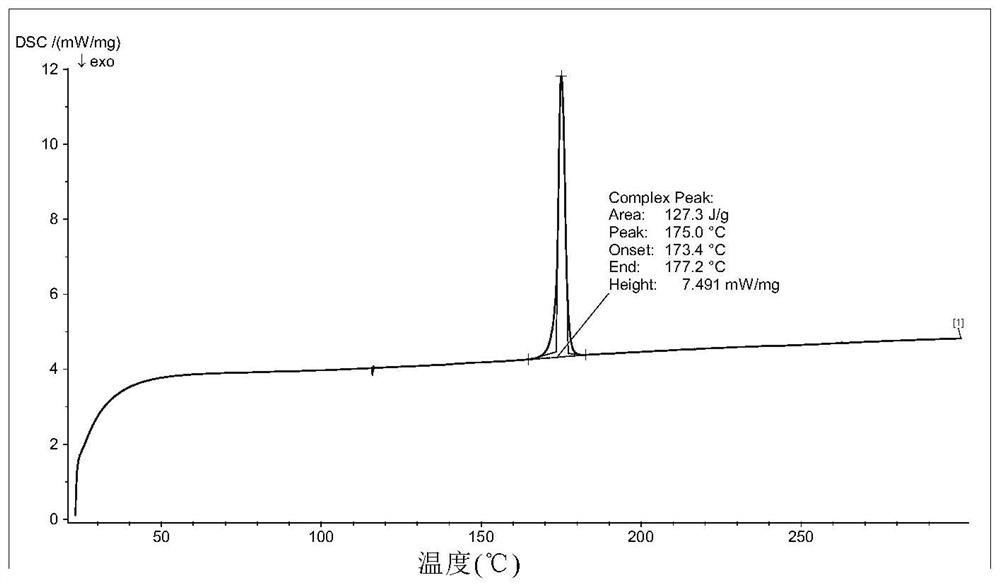
[0187] Take 500mg of 5-chloro-N 2 -(5-Methyl-4-(piperidin-4-yl)-2-((propan-2-yl-d 7 )oxy)phenyl)-N 4 -(2-((Propan-2-yl-d 7 )sulfonyl)phenyl)pyrimidine-2,4-diamine was added to the reaction flask, followed by 3 mL of ethanol and 1 mL of water. The obtained mixture was heated to 65°C, stirred and dissolved, and refluxed for 1 h; then 10 mL of water was added dropwise, a solid was precipitated, stirred for 0.5 h, then cooled to room temperature and filtered with suction. The filter cake was vacuum-dried to obtain a white solid, weighing 0.35 g, yield: 70%. Its X-ray powder diffraction pattern is Figure 1a same.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com