Application of MYOG gene as target point in preparation of drugs for treating cardiovascular diseases related to myocardial cell apoptosis
A technique for cardiomyocyte apoptosis and cardiovascular application in the field of biomedicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
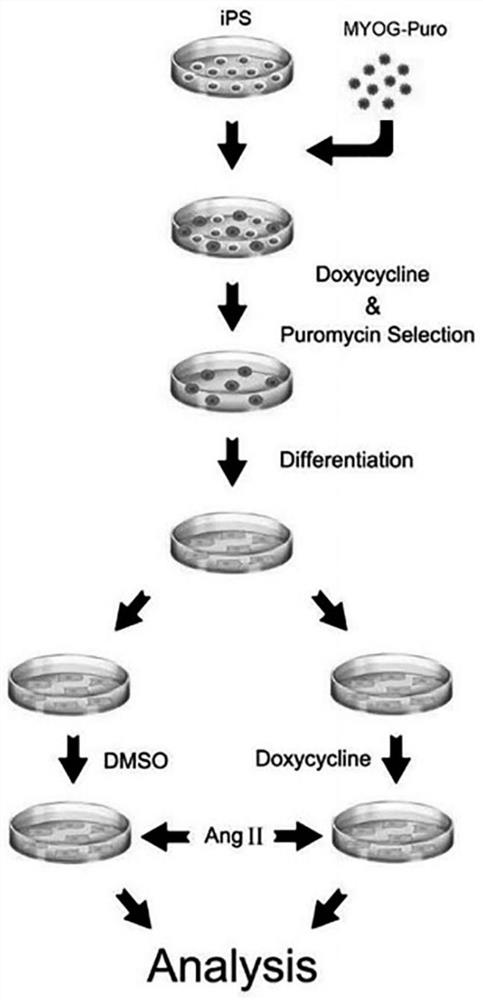
[0029] Example 1: Obtaining hIPSC-MYOG cell lines
[0030] 1.1 Construction of lentiviral expression vector: MYOG cDNA and a puromycin resistance gene were subcloned into pCW-Cas9-Blast vector (Addgene, 83481) by conventional molecular cloning methods, and Cas9 and Blast genes in the original vector were replaced to obtain pCW-MYOG.
[0031] 1.2 Lentiviral packaging
[0032] 1.2.1 Inoculate HEK293T cells into a 6-well plate, culture with D10 medium (DMEM medium + 10% fetal calf serum), and prepare for transfection when the cell confluence reaches 70%-80%.
[0033] 1.2.2 One hour before transfection, the original culture medium was discarded, and 2 mL / well of preheated serum-free OptiMEM culture medium was added.
[0034] 1.2.3 Perform transfection with Lipofectamine 2000 reagent according to the instructions. HEK293T cells were co-transfected with pCW-MYOG (20 μg), pVSVg (10 μg) (Addgene), and psPAX2 (15 μg) (Addgene).
[0035] 1.2.4 After 6 hours, replace the culture medi...
Embodiment 2
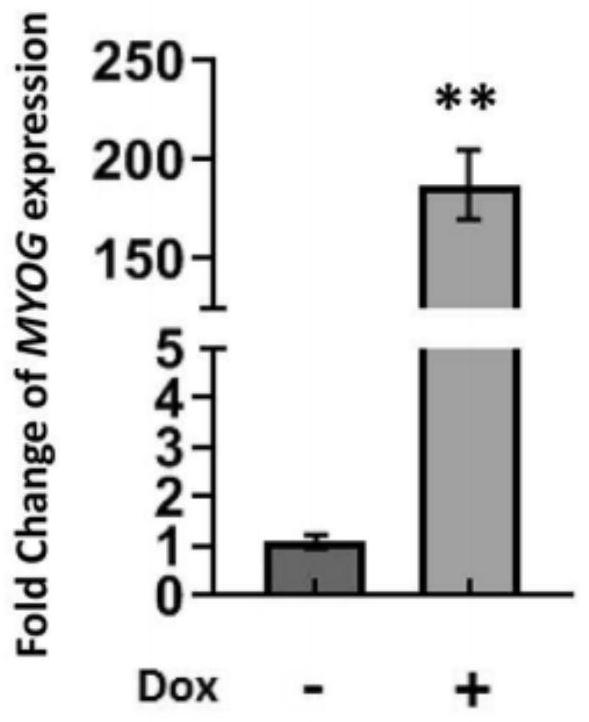
[0042] Example 2: Dox induces MYOG expression
[0043]2.1 Induction: Add doxycycline hydrochloride (Dox) (Sigma, D9891) at a final concentration of 2 μg / mL to STEMUP to induce the expression of MYOG, and use DMSO as a control. The control group has no DOX but only DMSO, marked as C1, and added to STEMUP; the test group has both DOX and DMSO, and DOX is first added to DMSO, marked as C2, and then added to STEMUP; the final concentration of DOX in STEMUP is 2 μg / mL; C1 The same as the addition of C2;
[0044] 2.2 Total RNA extraction: Total cellular RNA was extracted with UNlQ-10 Column Trizol Total RNA Extraction Kit (Sangon Biotech, B511321-0100). (The sample was treated with DNase I (deoxyribonuclease I, Sangon Biotech, B618252) for 30 minutes in advance);
[0045] 2.3 Reverse transcription: RNA was reverse-transcribed using the reverse transcription kit iScript Reverse Transcription Supermix (Bio-Rad, 1708841).
[0046] 2.4 qPCR detection of MYOG mRNA expression level: ac...
Embodiment 3
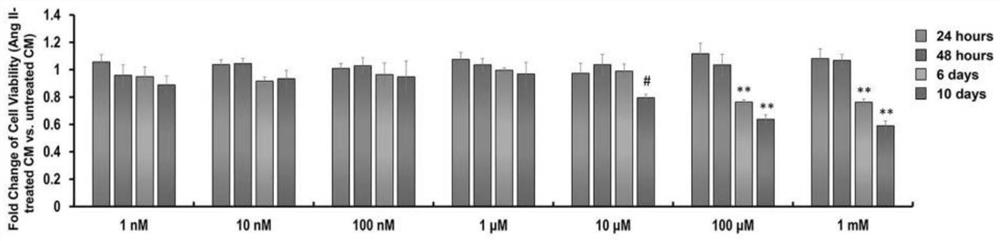
[0052] Example 3: Obtaining hiPSC-CM / hiPSC-MYOG-CM
[0053] 3.1 The differentiation of hiPSC: In RPMI-BSA medium [RPMI1640 medium (HyClone, SH30027.01) + 213 μg / mL AA2P (l-ascorbic acid 2-magnesium phosphate) (Sigma, A8960) and 0.1% bovine serum albumin (Sigma , A1470)], add the small molecule CHIR99021 (Tocris, 4423, the final concentration is 10mM)] to treat hiPSCs for 24 hours, and then change the RPMI-BSA medium and incubate for 48 hours. On the 4th day of differentiation, the small molecule IWP2 (Tocris, 3533, final concentration 5 μM) was added to the RPMI-BSA medium to treat the cells. After 48h, the medium was replaced with RPMI-BSA medium. At this stage, it differentiates from hipsc to hipsc-cm. In subsequent experiments, cardiomyocytes were cultured with RPMI1640 medium plus 3% serum replacement (Gibco, 10828-028).
[0054] 3.2 Purification of hiPSC-MYOG-CM: hiPSC-CM was purified using a metabolic selection method. The metabolic selection medium was DMEM medium (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com