Refining method of osimertinib
A refining method and crude product technology, applied in the direction of organic chemistry, can solve problems such as low production efficiency, high cost, and inconspicuous effect, and achieve the effect of improving purity and reducing residue
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
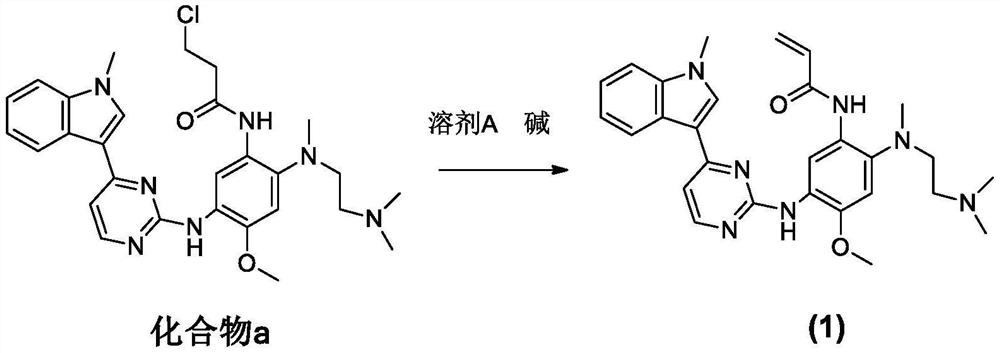
[0050] In one of the embodiments, the preparation method of the crude ostinib comprises the following steps:
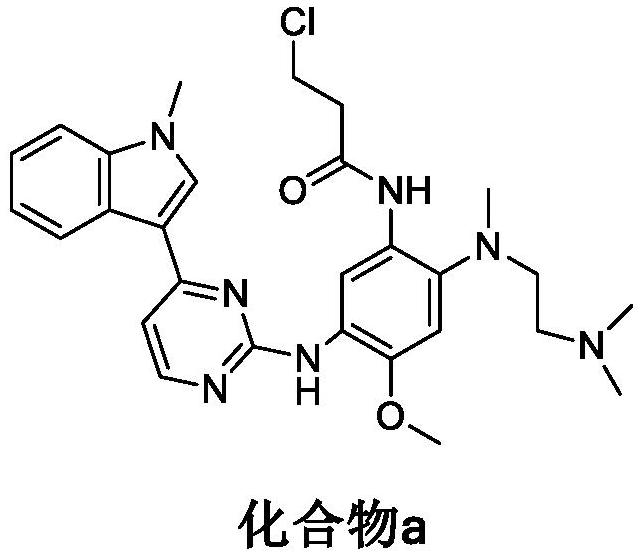
[0051] Compound a, solvent A and base are mixed and reacted;
[0052]
[0053] The alkali is selected from one or more of sodium hydroxide, potassium hydroxide, sodium methylate and potassium tert-butoxide;
[0054] The solvent is selected from one or more of acetone, dimethyl sulfoxide and tetrahydrofuran;
[0055] The reaction temperature of the reaction is 60°C to 100°C.
[0056] In one of the embodiments, after the step of cooling down to precipitate crystals, an operation of filtering is also included. Preferably, suction filtration is used for solid-liquid separation, and the filter cake and filtrate are collected separately. In order to further improve the purity of ostinib, for the same batch of crude product, after refining once, the refining method of the present invention can be repeated 2 to 3 times for the obtained product. In order to increase the...
Embodiment 1
[0060] This embodiment provides a kind of ostinib and its refining method, the steps are as follows:
[0061] Step 1) Synthetic Ostinib crude product:
[0062] Add 200 g of compound a, 23 g of sodium hydroxide and 600 mL of acetonitrile into a three-necked flask, and react at 80° C. for 10 h to obtain a crude ostinib, which has a purity of 98.1% as detected by HPLC.
[0063]
[0064] Step 2) refinement of crude product:
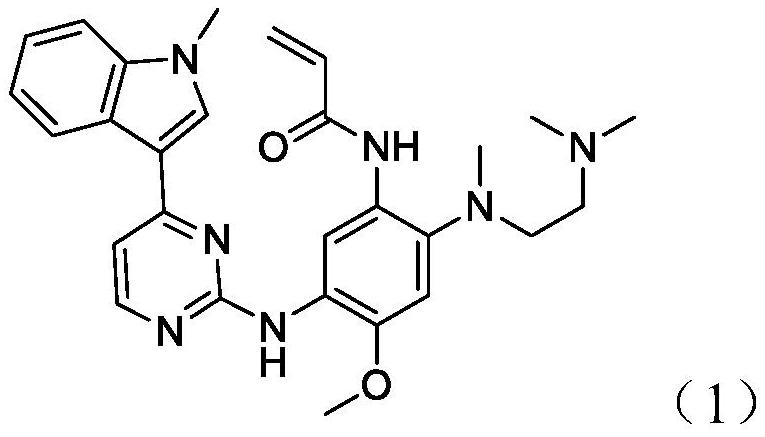
[0065] Take 200 g of the crude ostinib prepared in step 1), add it to 200 mL of tetrahydrofuran, heat to 60°C to dissolve the crude ostinib, then cool to 5°C at a rate of 3°C / min, crystallize for 1 hour, and filter with suction , dried under reduced pressure at 50° C. to obtain 190 g of the refined product of white crystals, which is the refined product of ostinib, and the yield is 95%.
Embodiment 2
[0067] This example provides an ostinib and its refining method, the steps of which are basically the same as in Example 1, the main difference being that the organic solvent used in the refining process is 2-butanone.
[0068] Step 1) Synthetic Ostinib crude product:
[0069] Add 200 g of compound a, 23 g of sodium hydroxide and 600 mL of acetonitrile into a three-necked flask, and react at 80° C. for 10 h to obtain a crude ostinib, which has a purity of 98.1% as detected by HPLC.
[0070]
[0071] Step 2) refinement of crude product:
[0072] Take 200g of crude ostinib prepared in step 1), add it to 200mL of 2-butanone, heat to 60°C to dissolve the crude ostinib, then cool to 5°C at a rate of 3°C / min, and crystallize for 1h , suction filtration, and dried under reduced pressure at 50° C. to obtain 185 g of refined products of white crystals, which is the refined product of ostinib, and the yield is 92.5%. The purity of the refined product of ostinib detected by HPLC is 9...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com