Nlrp3 inflammasome inhibitors
A -NH2, C3-C5 technology, used in anti-inflammatory agents, non-central analgesics, allergic diseases, etc., can solve problems such as poor metabolic stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 9a
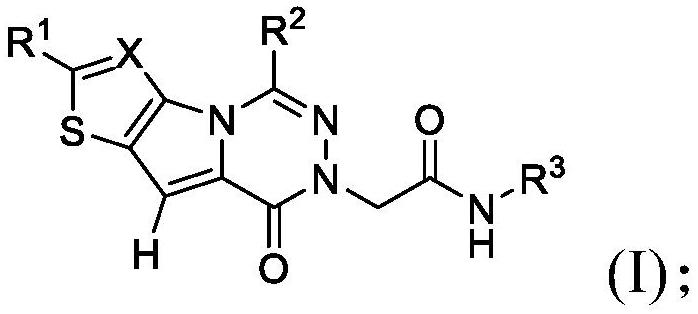
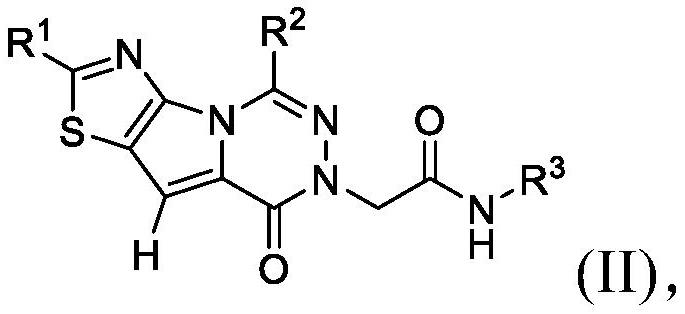
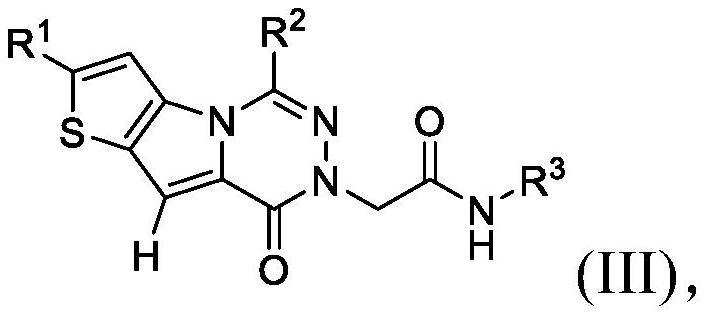
[0100] In embodiment 9a, and embodiment 9, the invention relates to a compound having any one of formulas (I) to (III), or a pharmaceutically acceptable salt thereof, wherein R 3 Choose from the following:
[0101]
[0102] where R 3e independently selected from -OH, C 1 -C 4 Alkyl, C 3 -C 6 Cycloalkyl, halogenated, -OC(O)(C 1 -C 4 Alkyl), halogenated C 1 -C 4 Alkyl, -C(O)O(C 1 -C 4 Alkyl), hydroxyl C 1 -C 4 Alkyl, C 1 -C 4 Alkoxy, halo C 1 -C 4 Alkoxy, -NH 2 、-SO 2 NH 2 、-SO 2 NH(C 1 -C 4 Alkyl), -NHSO 2 (C 1 -C 4 Alkyl), -NH(C 1 -C 4 Alkyl), -C(O)NH 2 , CO 2 H; and
[0103] R 3g Choose from H, C 1 -C 4 Alkyl, C 3 -C 6 Cycloalkyl, Halo and HaloC 1 -C 4 alkyl.
[0104] In embodiment 9b, and according to embodiment 9a, the invention relates to a compound having any of formulas (I) to (III), or a pharmaceutically acceptable salt thereof, wherein R is preferred 3e selected from the group consisting of: halo, OH, CH 3 、CH 2 OH, -NHS(O) 2...
example
[0450] Example of the invention
[0451] The disclosure is further illustrated by the following examples and synthetic schemes, which should not be construed to limit the scope or spirit of the disclosure to the specific procedures described herein. It should be understood that these examples are provided to illustrate certain embodiments and are not intended to limit the scope of the present disclosure thereby. It should be further understood that various other embodiments, modifications and their equivalents that can occur to those skilled in the art themselves can be employed without departing from the spirit of the present disclosure and / or the scope of the appended claims.
[0452] The compounds of the present disclosure can be prepared by methods known in the art of organic synthesis. In all methods, it is understood that protecting groups for sensitive or reactive groups may be used where necessary according to general chemistry principles. Protective groups were ma...
example 1
[0688] 2-(2-Chloro-5-isopropyl-8-oxothieno[2',3':4,5]pyrrolo[1,2-d][1,2,4]triazine-7 (8H)-yl)-N-(pyrimidin-4-yl)acetamide
[0689]
[0690] A solution of Intermediate 5 (70 mg, 0.261 mmol) in DMF (5 mL) was cooled to 0 °C in an ice bath and LiHMDS (1M in THF, 0.680 mL, 0.680 mmol) was added dropwise. The mixture was stirred at 0 °C for 20 min, then a solution of 2-chloro-N-(pyrimidin-4-yl)acetamide (65.3 mg, 0.314 mmol) in DMF (1 mL) was added slowly. The ice bath was removed and the solution was stirred overnight at RT. The reaction mixture was partitioned between EtOAc and water. The organic layer was washed with Na 2 SO 4 Dry, filter and evaporate. The residue was purified by preparative RP chromatography to afford the title compound as a white solid. 1 H NMR (400MHz, DMSO-d6) δ (ppm) 11.24 (s, 1H), 8.92 (d, 1H), 8.67 (d, 1H), 8.00 (dd, 1H), 7.82 (s, 1H), 7.50 ( s,1H), 4.90(s,2H), 3.62(sept,1H), 1.31(d,6H). LC-MS: Rt=1.0min; MS m / z[M+H] + 403.2
[0691] Simi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com