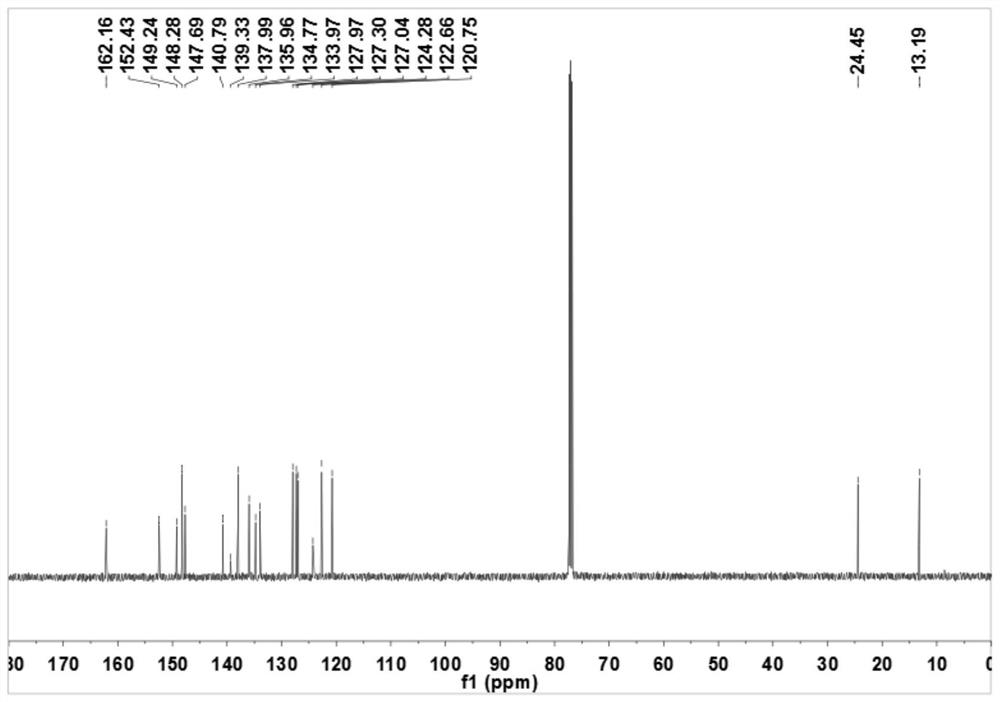
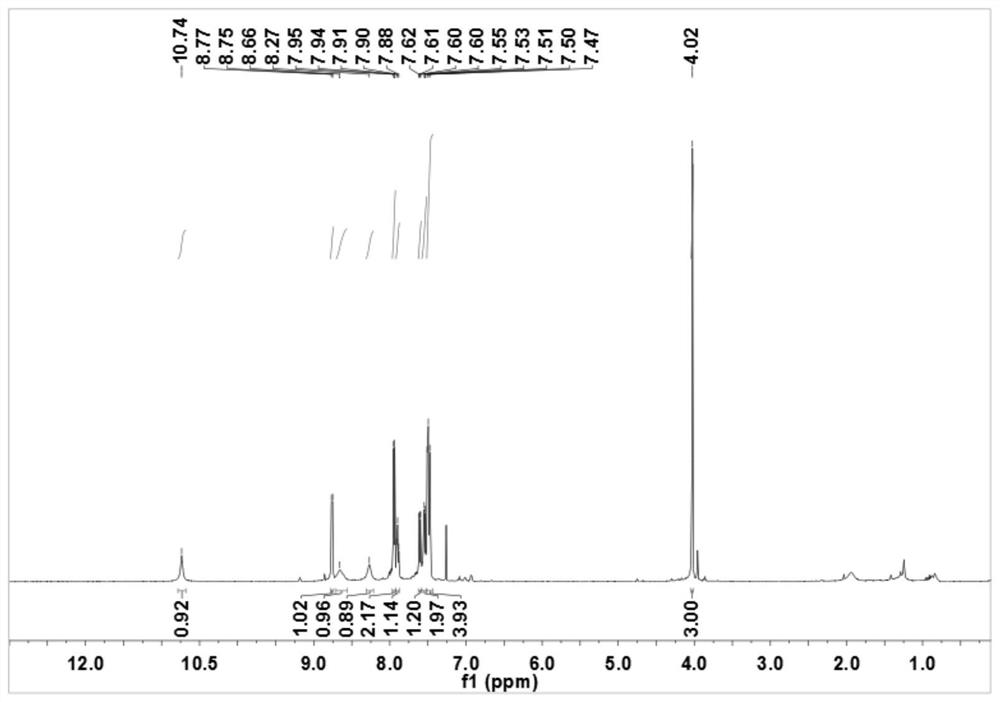
Sulfonyl pyridine amide derivatives and preparation method thereof
A technology for sulfonyl pyridine amides and derivatives, which is applied in the field of sulfonyl pyridine amide derivatives and their preparation, can solve problems such as reaction conditions restricting practicability, and achieve scientific and reasonable synthesis methods, industrialization promotion, and efficient recycling capabilities. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0046] Preparation of Chitosan-supported Copper-Carbon Nanocatalysts
[0047] In a 100 mL round bottom flask equipped with a reflux condenser and a magnetic stir bar, anhydrous copper acetate (90.83 mg, 0.500 mmol) was dissolved in H 2 O (40mL). Chitosan (690 mg) was then added to the solution to obtain a suspension which was stirred at 50 °C for 3 h. After the solution was cooled to room temperature, the H was slowly removed by suction filtration under reduced pressure. 2 O, the filter cake was washed three times with ethanol and water to give a light blue solid. The light blue solid obtained was dried at 60° C. under vacuum for 12 hours. The dried samples were evenly transferred into porcelain boats and then put into a tube furnace. The tube furnace was evacuated and then flushed with nitrogen for half an hour. Keep nitrogen flowing in the tube furnace, heat the tube furnace to 200 °C with a temperature gradient of 2 °C / min, and keep it in nitrogen atmosphere for 2 hour...
Embodiment 1
[0049] The preparation of N-(2-ethylphenyl) pyridine-2-carboxamide (R in structural formula II 1 is pyridyl, R 2 Ortho-substituted ethyl)
[0050]In a 150mL round-bottomed flask, add 2-pyridinium formate (12mmol, 1.48g), 2-ethylaniline (10mmol, 1.21g) and 50mL of dichloromethane (DCM), stir in an ice bath, and then pour into the round-bottomed flask DCM solution mixed with dicyclohexylcarbodiimide (DCC) (15mmol, 3.1g) and 4-dimethylaminopyridine (DMAP) (5mmol, 0.61g) was added dropwise, and the reaction was continued at room temperature after the dropwise addition was completed. Hours, the reaction was checked by TLC. After the reaction is completed, the reaction solution is suction-filtered, the filtrate is collected, spin-dried, and the eluent is purified through the column with the ratio of petroleum ether and ethyl acetate (25:1), and N-(2-ethyl phenyl)pyridine-2-carboxamide.
[0051] The preparation of pyridine-3-sodium sulfinate (R in the formula III 3 for meta-subs...
Embodiment 2
[0061] The preparation of N-(2-methoxyphenyl) pyridine-2-carboxamide (R in structural formula II 1 is pyridyl, R 2 for ortho methoxy)
[0062] In a 150mL round bottom flask, add 2-pyridinium formate (12mmol, 1.48g), 2-methoxyaniline (10mmol, 1.23g) and 50mL of dichloromethane (DCM), stir under ice bath, and then pour A DCM solution mixed with dicyclohexylcarbodiimide (DCC) (15mmol, 3.1g) and 4-dimethylaminopyridine (DMAP) (5mmol, 0.61g) was added dropwise in the flask, and the reaction was continued at room temperature after the addition was complete After 6 hours, the reaction was checked by TLC. After the reaction is completed, the reaction solution is suction-filtered, the filtrate is collected, spin-dried, and the eluent is purified by column with the ratio of petroleum ether and ethyl acetate (25:1), and N-(2-methoxy phenyl)pyridine-2-carboxamide.
[0063] The preparation of N-[4-(benzenesulfonyl)-2-methoxyphenyl]pyridine-2-carboxamide (R in structural formula I 1 is...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com