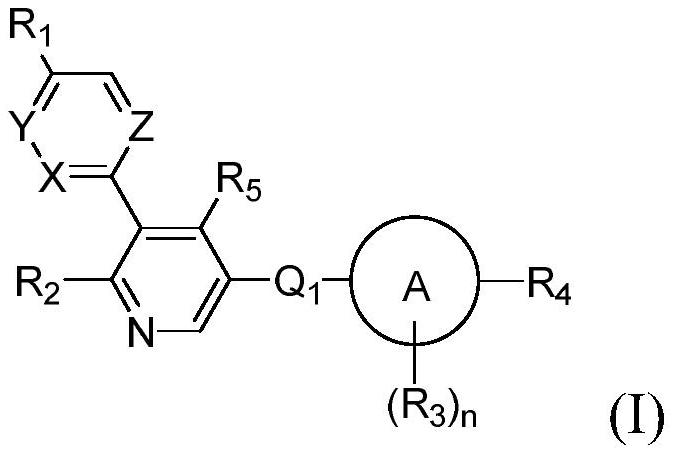
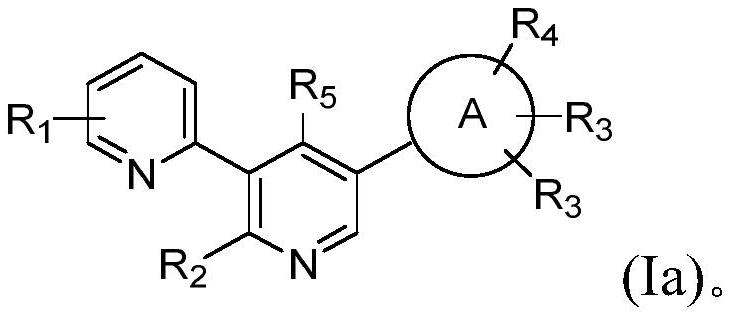
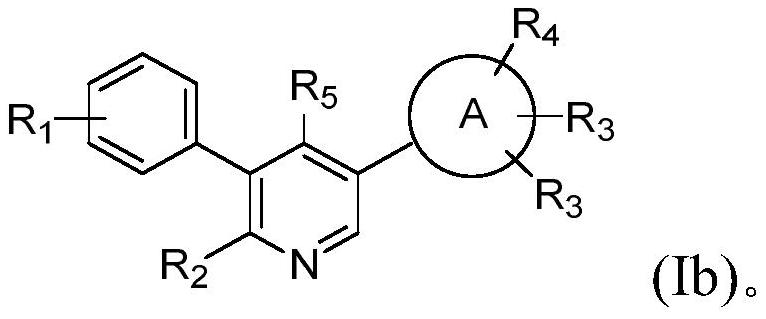
Aryl-bipyridine amine derivatives as phosphatidylinositol phosphate kinase inhibitors
An aryl and heteroaryl technology, applied to compounds containing elements of group 3/13 of the periodic table, drug combinations, medical preparations containing active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0715] Compound preparation
[0716] The compounds of the present invention can be prepared in a variety of ways well known to those skilled in the art of organic synthesis. For example, the compounds of the present invention can be synthesized using the methods described below, as well as synthetic methods known in the art of synthetic organic chemistry, or variations thereof known to those skilled in the art. Preferred methods include, but are not limited to, those described below. Compounds of the present invention can be synthesized by following the steps outlined in general scheme 1, which include the assembly of intermediates or different sequences of compound (II). Starting materials are commercially available or prepared by methods known in the reported literature or as shown below.
[0717] Compounds of formula (I) can be obtained (Scheme 1) by starting from, for example, compounds of formula (II), where T represents a coupling metal, including but not limited to b...
Embodiment 1
[0777] Example 1: 4-([3,4'-bipyridyl]-5-yl)-N,N-dimethylbenzamide
[0778]
[0779] in N 2 Down to 3,5-dibromopyridine (200mg, 0.84mmol), 4-pyridylboronic acid (52mg, 0.42mmol) and K 2 CO 3 (0.47g, 3.38mmol) in 1,4-dioxane (3ml) and water (0.75ml) was added Pd(PPh 3 ) 4 (49 mg, 0.04 mmol). The resulting mixture was stirred at 70 for 3 hours, then [4-(dimethylcarbamoyl)phenyl]boronic acid (81 mg, 0.42 mmol) was added, and the mixture was stirred at 90 for 2 hours. More [4-(dimethylcarbamoyl)phenyl]boronic acid (81 mg, 0.42 mmol) and PdCl2 (Amphos) (15 mg, 0.02 mmol) were added and stirring was continued at 90 overnight. When cooled to room temperature, water and EtOAc were added, the organic layer was separated, and the aqueous layer was extracted with EtOAc. The combined organics were washed with brine, washed with Na 2 SO 4 Drying, filtration, concentration, and purification by preparative HPLC afforded the product as a solid (18 mg, 7%). 1 H NMR (500MHz, chlorofo...
Embodiment 2
[0780] Example 2: 4-(5-(1H-pyrrolo[2,3-b]pyridin-4-yl)pyridin-3-yl)-N,N-dimethylbenzamide
[0781]
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com