Active free radical polymerization method with zinc phthalocyanine dye as near-infrared light catalyst
A polymerization method and near-infrared light technology, applied in the field of living radical polymerization, can solve the problems of not utilizing the photoconversion performance of phthalocyanine compounds, and the reaction products do not have "activity"
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
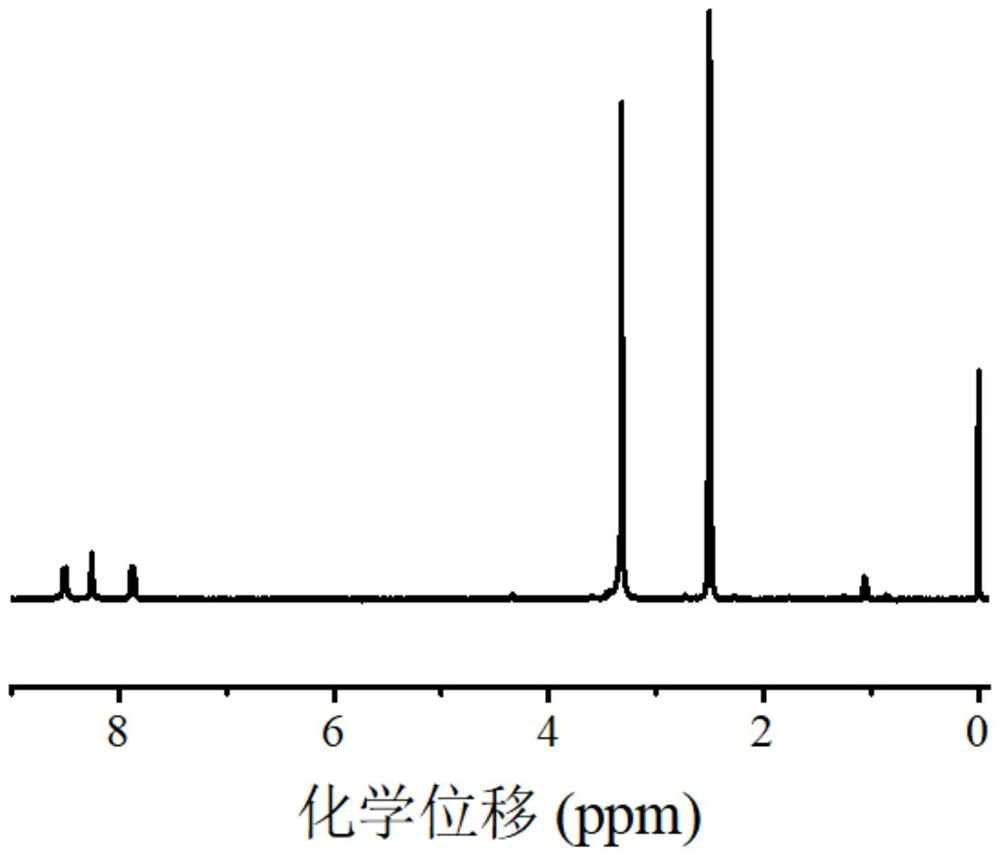
[0078] Example 1 Synthesis of polymeric zinc phthalocyanine dye near-infrared photocatalyst ZnTAPc-MAm
[0079] Dissolve zinc tetraaminophthalocyanine dye (ZnTAPc) (0.32g, 0.50mmol) in 20mL of DMF, add TEA (83.4μL, 0.60mmol), stir on a magnetic stirrer, and then dropwise add methacryloyl chloride (44.8 μL, 0.55mmol) mixed with anhydrous dichloromethane (10mL), stirred overnight at room temperature. Then the reaction solution was poured into 150 mL of water, and the solid product was separated and collected by suction filtration, and further purified by washing with 1.0 M hydrochloric acid and 1.0 M sodium hydroxide, respectively. Finally, the product was washed with deionized water and precipitated using 10000r min -1 Centrifuge at a high speed. The obtained dark blue-black product was used after being dried in a vacuum oven. The reaction scheme is as follows:
[0080]
Embodiment 2
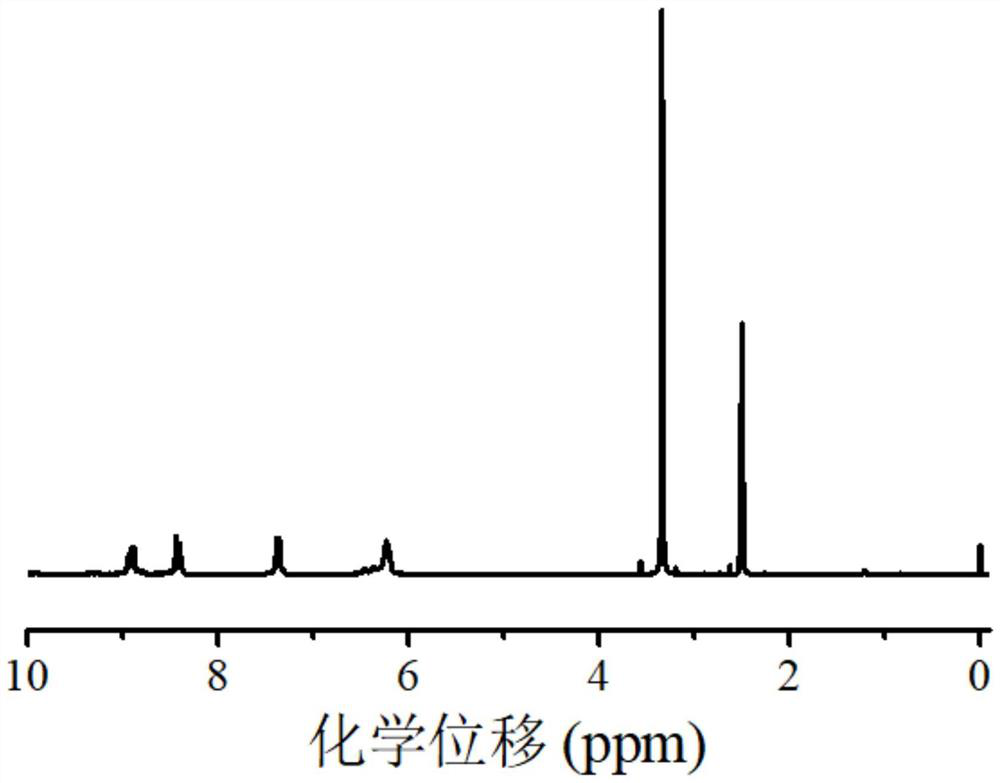
[0081] Example 2 Synthesis of polymeric zinc phthalocyanine dye near-infrared photocatalyst ZnTAPc-Am
[0082] Disperse ZnTAPc (0.32g, 0.50mmol) in 20mL of DMF, add TEA (83.4μL, 0.60mmol), stir on a magnetic stirrer, then add dropwise acryloyl chloride (53.2μL, 0.55mmol) and anhydrous dichloro Methane (10 mL) mixed solution was stirred at room temperature overnight. Then the reaction solution was poured into 150 mL of water, and the solid product was separated and collected by suction filtration, and further purified by washing with 1.0 M hydrochloric acid and 1.0 M sodium hydroxide, respectively. Finally, the product was washed with deionized water and precipitated using 10000r min -1 Centrifuge at a high speed. The dark blue-black product obtained is the acrylamide zinc phthalocyanine dye monomer (ZnTAPc-Am) and dried in a vacuum oven with a product yield of 80.2%. The reaction scheme is as follows:
[0083]
Embodiment 3
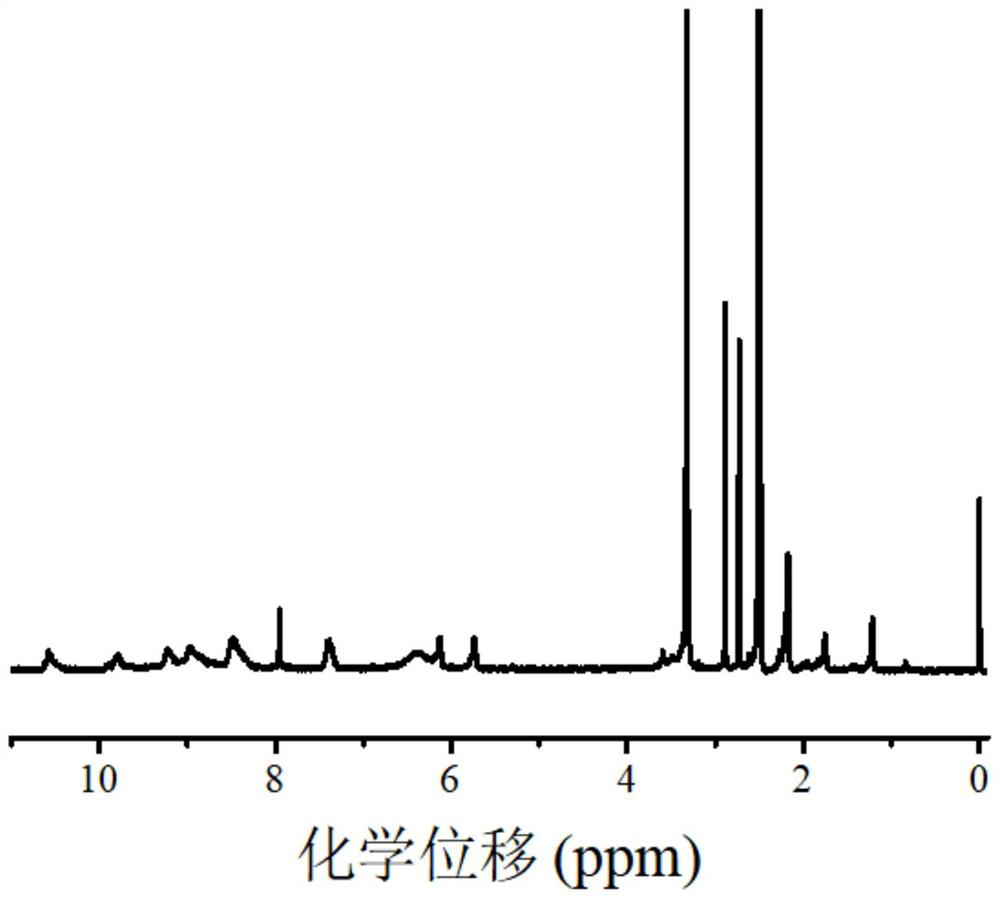
[0084] Example 3 Synthesis of polymeric zinc phthalocyanine dye near-infrared photocatalyst ZnTAPc-A
[0085] (1) Synthesis of acid chloride compound (acrylic acid-3-chloro-3-oxopropyl ester): acid chloride 3-(acryloxy)propionic acid (79.30mg, 0.55 mmol) with thionyl chloride (5.0mL) , dropwise added two drops of DMF as a catalyst, stirred for 30 minutes, removed excess thionyl chloride by rotary evaporation, sealed and kept for the next step of reaction. The reaction scheme is as follows:
[0086]
[0087] (2) Synthesis of ZnTAPc-A: Dissolve ZnTAPc (0.32g, 0.50mmol) in 20mL of DMF, add TEA (83.4μL, 0.60mmol), stir on a magnetic stirrer, and then dropwise add acrylic acid-3-chloro- A mixed solution of 3-oxopropyl ester (89.40 mg, 0.55 mmol) and anhydrous dichloromethane (10.0 mL) was stirred overnight at room temperature. Then the reaction solution was poured into 150 mL of water, and the solid product was collected by suction filtration, and further washed and purified w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com