Dissociation method of antigen in aluminum adjuvant-adsorbed novel coronavirus inactivated vaccine
A coronavirus and inactivated vaccine technology, applied in the field of biopharmaceuticals, to achieve the effect of solving the instability of biological structure, good repeatability, and destroying the adsorption force of aluminum adjuvants
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] This embodiment provides a method for dissociation of antigens in an aluminum adjuvant-adsorbed novel coronavirus inactivated vaccine, which specifically includes the following steps:
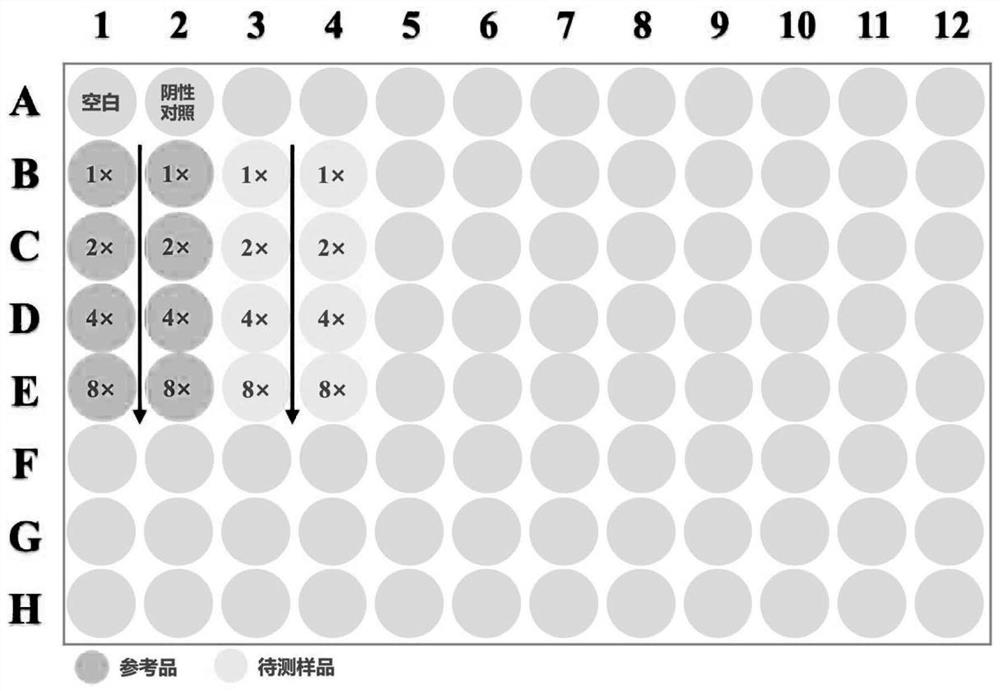
[0044] (1) Preparation of test samples and reference products of aluminum adjuvant-adsorbed novel coronavirus inactivated vaccine:
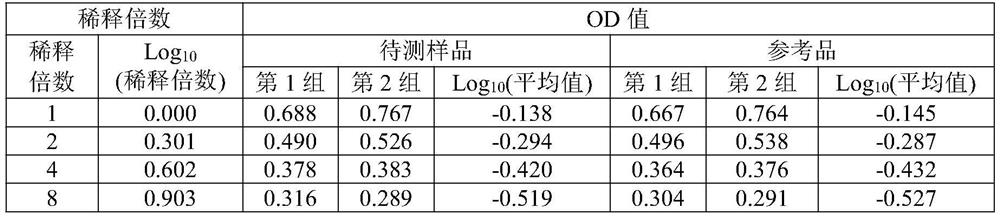
[0045] The sample to be tested for the aluminum adjuvant-adsorbed novel coronavirus inactivated vaccine is self-made (currently there is no commercial product). The preparation scheme is as follows: the virus stock solution of the novel coronavirus inactivated vaccine (in the detection index of the virus stock solution, the antigen content is 138U / ml, and the protein content is 215μg / ml) is diluted in proportion according to the target concentration of the prepared 8U / ml antigen, and then added with aluminum The adjuvant prepared the aluminum adjuvant adsorption type SARS-CoV-2 inactivated vaccine test sample with a final concentration of novel coronavirus ...
Embodiment 2
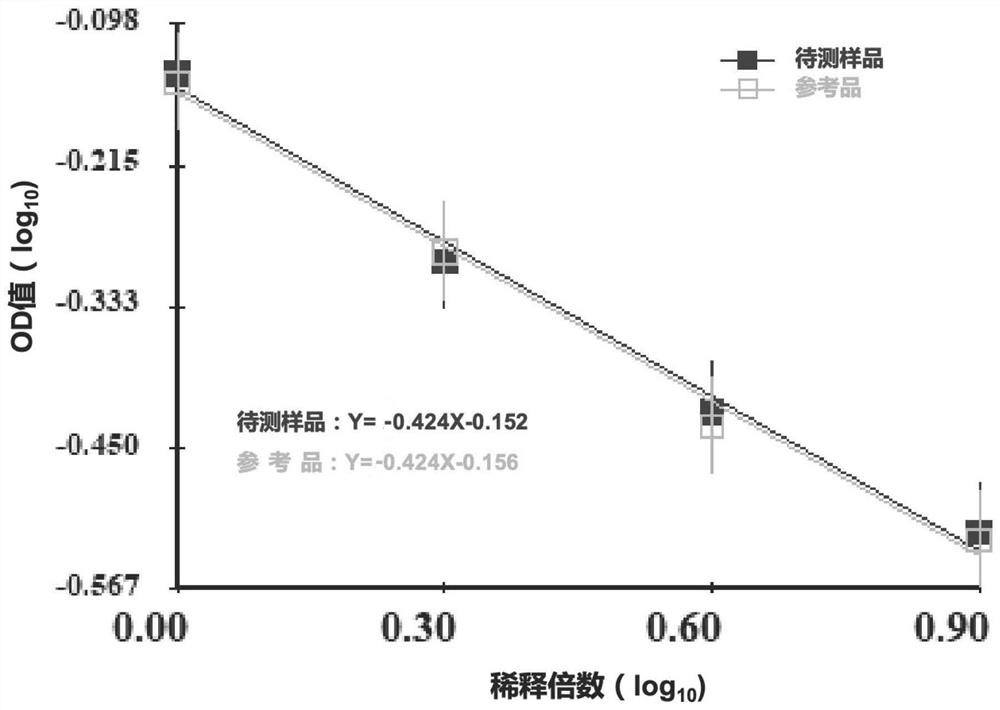
[0065] In this example, the results of antigen dissociation in SARS-CoV-2 inactivated vaccine samples with different quality attributes are provided, and the applicability of the dissociation method to different samples is investigated. Specifically include:
[0066] (1) Preparation of samples to be tested and reference products:
[0067] The preparation process of the sample to be tested is the same as in Example 1, and the concentration preparation is shown in Table 3, and the corresponding reference product is prepared, and the preparation method is the same as in Example 1.
[0068] (2) Preparation of desorbent composition: same as Example 1.
[0069] (3) Implementation process of the dissociation program: the same as in Example 1.
[0070] (4) The detection method of the antigen content in the novel coronavirus antigen desorption solution is the same as in Example 1, and see Table 3 for the specific dilution method.
[0071] Table 3 shows the detection results of SARS-...
Embodiment 3
[0077] This example provides the results of dissociation of antigens in SARS-CoV-2 inactivated vaccines using dissociation methods with different concentrations of desorbent compositions and different dissociation program parameter conditions, and investigates the applicability of the method to samples. Specifically include:
[0078] (1) Preparation of test sample and reference product: same as Example 1.
[0079] (2) Preparation of desorbent composition:
[0080] The final mass concentration of sucrose is 5%-10% (w / v).
[0081] The mass final concentration of magnesium chloride hexahydrate is 0.5%-1% (w / v).
[0082] 0.6mol / L Potassium Phosphate Buffer Preparation Method:
[0083] It is obtained by mixing 1mol / L potassium phosphate buffer solution and water for injection in proportion. Take the preparation of 10ml, 0.6mol / L potassium phosphate buffer solution as an example, measure 6ml, 1mol / L potassium phosphate buffer solution and 4ml water for injection, and after mixin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com