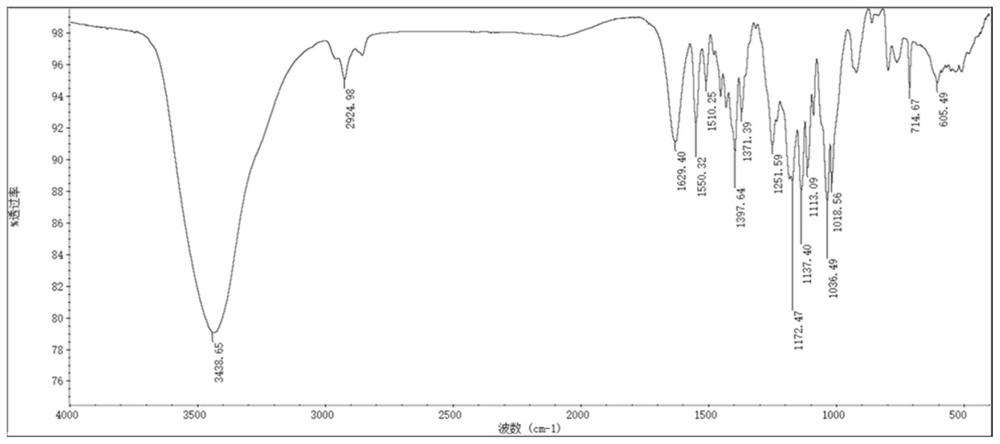
Heptamethine carboxyl indocyanine dye, preparation method and application thereof
A technology of heptamethine carboxyl and indole cyanine, applied in the direction of methine/polymethine dyes, organic dyes, chemical instruments and methods, etc., can solve the problems of large consumption of organic solvents, high price, low purity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0148] As mentioned above, the present application relates to a preparation method of heptamethine carboxy indole cyanine dye, comprising the following steps: reacting 2,3,3-trimethyl-carboxy indole derivatives with nucleophilic substitution compounds to obtain organic Ammonium salt: mix organic ammonium salt and cycloalkene derivatives in an environment-friendly organic solvent for reaction, add an organic precipitant to the product after cooling and let it stand overnight to obtain the heptamethene carboxyindole cyanine dye. The method has the advantages of short synthesis route, simple process, no catalyst, high yield, simple purification method, high atom utilization rate and less consumption of organic solvents, can greatly improve the preparation efficiency of such dyes, and realize low-cost mass production , It is of great significance in the production and application research of heptamethine carboxyindole cyanine dyes.
[0149] In addition, the preparation method of t...
Embodiment 1
[0156] Example 1 Synthesis of 2,3,3-trimethyl-4-carboxyindole
[0157] Dissolve p-carboxyphenylhydrazine, 3-methyl-2-butanone, and anhydrous sodium acetate at a molar ratio of 1:1.1:1.5 in acetic acid, and react under reflux and stirring for 8 hours. The reaction solvent was removed by rotary evaporation, and then a mixed solution of water and methanol with a volume ratio of 9:1 was added to dissolve the remaining substances. The resultant was filtered, and then exposed to crystallization at room temperature for 48 hours to obtain crystal 2,3,3-trimethyl-4-carboxyindole.
Embodiment 2
[0158] Embodiment 2 synthetic compound 53
[0159] Compound 53 was synthesized according to the following route:
[0160]
[0161] 1) Synthesis of 2,3,3-trimethyl-1-(butane)-carboxyindole
[0162] The 2,3,3-trimethyl-carboxyindole and 4-bromobutane obtained in Example 1 were added into the reactor at a molar ratio of 1:1.5, and the reactor was sealed and then evacuated to 10 Pa. The reaction system was heated to 110° C. and stirred for 8 hours, then cooled to room temperature. The obtained product was filtered with suction and used directly for the next reaction.
[0163] 2) Synthesis and purification of compound 53
[0164] Add 2-chloro-1-formyl-3 to the reactor in a molar ratio of 1:2.5 to 2,3,3-trimethyl-1-(butane)-carboxyindole obtained in step 1) -Hydroxymethylenecyclopentene. After completely dissolving with methanol, the reaction system was heated to 75° C. for 24 hours under closed conditions, then cooled to room temperature, and placed in a 4° C. refrigerator ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com