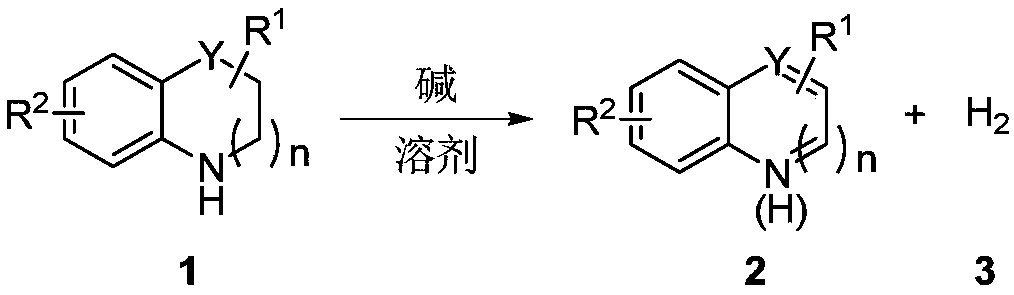
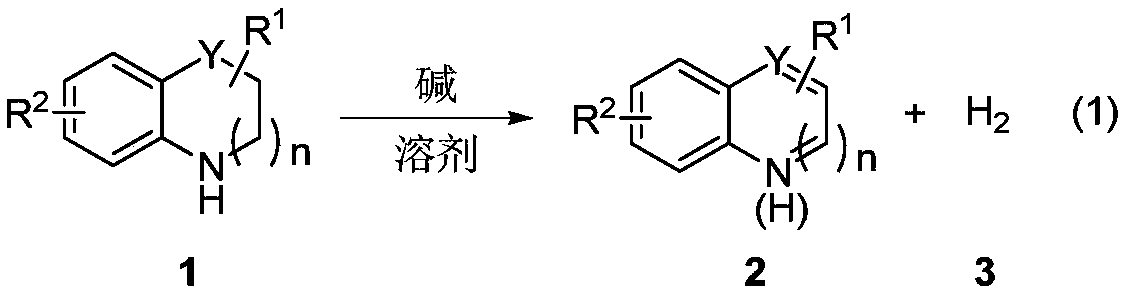
Method for preparing hydrogen by promoting dehydrogenation of nitrogen heterocyclic compound through alkali
A nitrogen heterocyclic compound, dehydrogenation technology, applied in chemical instruments and methods, hydrogen, inorganic chemistry and other directions, can solve problems such as increasing the reaction cost, and achieve the effects of simple operation, wide application range and high reaction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032]
[0033] The specific process is: Weigh the saturated six-membered nitrogen heterocyclic compound 1a (0.5mmol), base tBuOK (1.5mmol), add to a 25mL reaction tube with a branch, after nitrogen replacement, add 2mL o-xylene, put The reaction was carried out in an oil bath at 140°C for 36 hours, and the hydrogen gas generated during the reaction was collected by a balloon. After the reaction is complete, cool to room temperature, filter with diatomaceous earth, and rotate under reduced pressure to remove the solvent, and then use silica gel column chromatography (eluent is petroleum ether (60-90°C) / ethyl acetate: 4:1, v / v), the light yellow liquid product 2a (59 mg, yield 90%) was obtained. The target product was confirmed by NMR spectroscopy. The generation of hydrogen gas was detected by gas phase, with CH 4 As an internal standard, 19.1 mL of hydrogen gas was generated.
Embodiment 2
[0035] The reaction steps and operations are the same as in Example 1, except that the base tBuONa (1.5 mmol) is added to the reaction system. The reaction was stopped, and the target product 2a (51 mg, yield 76%) was obtained as a light yellow liquid after post-processing. The generation of hydrogen gas was detected by gas phase, with CH 4 As an internal standard, generate 15.7mL of hydrogen gas.
Embodiment 3
[0037] The reaction steps and operations are the same as in Example 1, except that the base tBuOLi (1.5 mmol) is added to the reaction system. The reaction was stopped, and the target product 2a (46 mg, yield 70%) was obtained as a light yellow liquid after post-processing. The generation of hydrogen gas was detected by gas phase, with CH 4 As an internal standard, 14.6 mL of hydrogen gas was generated.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com