Indenofluoranthene compound and application thereof
A technology of indenofluoranthene and compound, applied in the field of organic electroluminescence display
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0050] According to the preparation method provided by the present invention, those skilled in the art can use known common means to realize, such as further selecting a suitable catalyst and solvent, determining a suitable reaction temperature, time, etc., which are not particularly limited in the present invention. The solvents, catalysts, bases and other raw materials used in the preparation process can be synthesized through open commercial channels or methods known in the art.
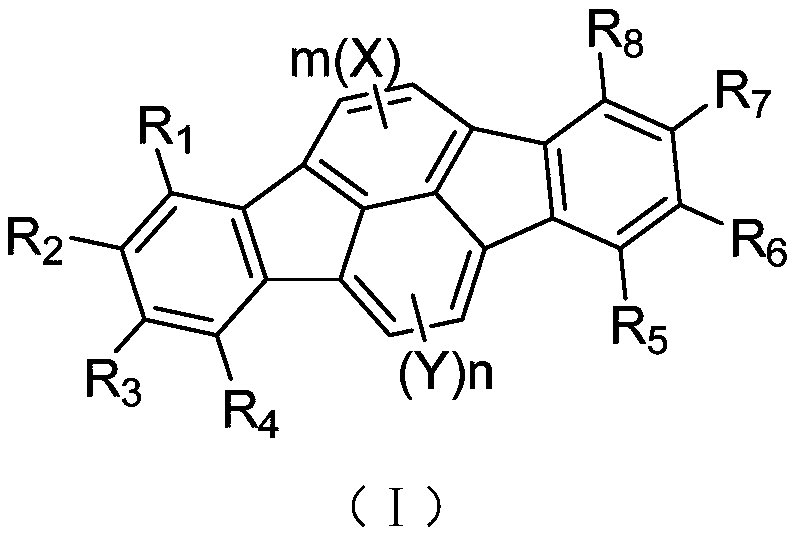
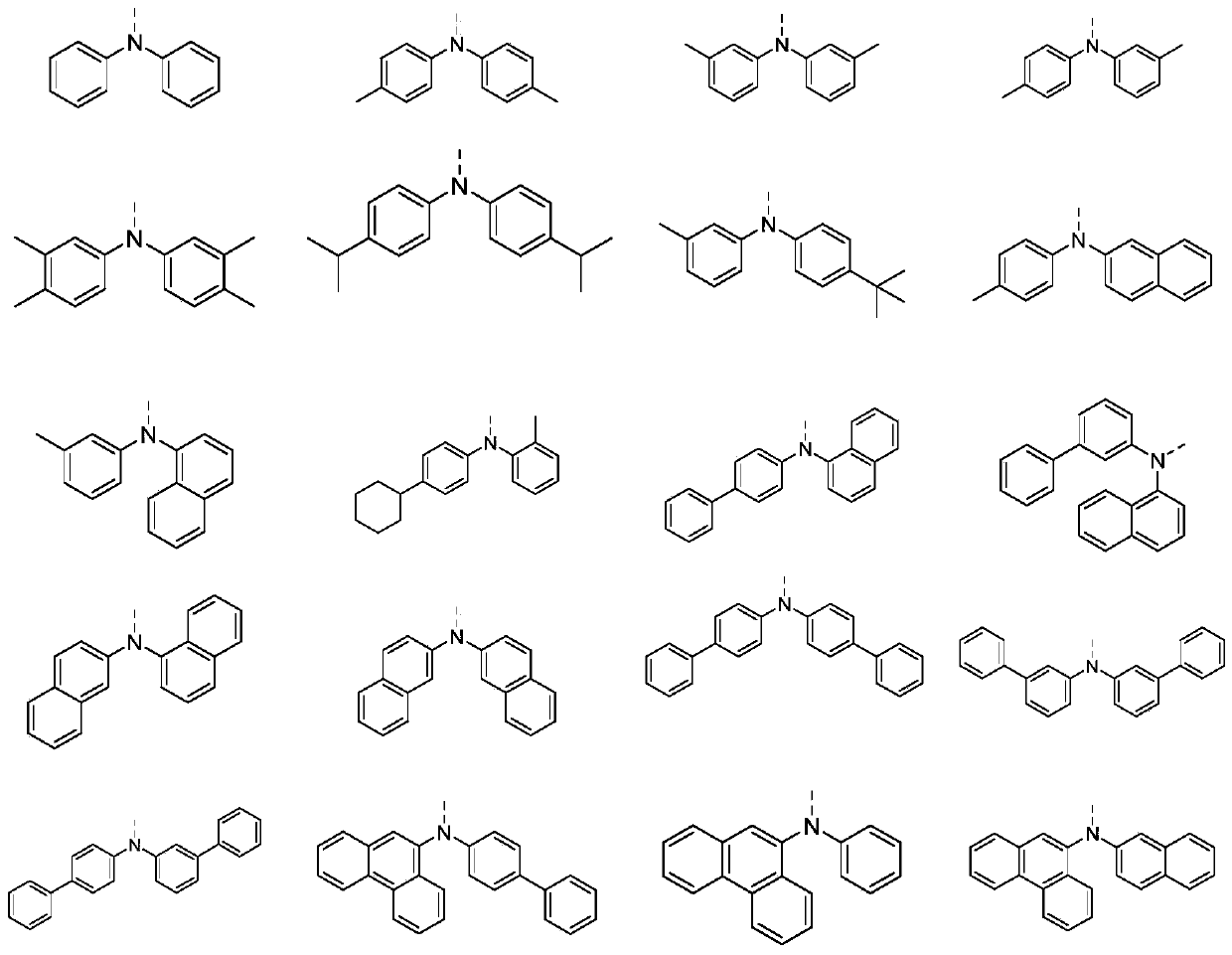
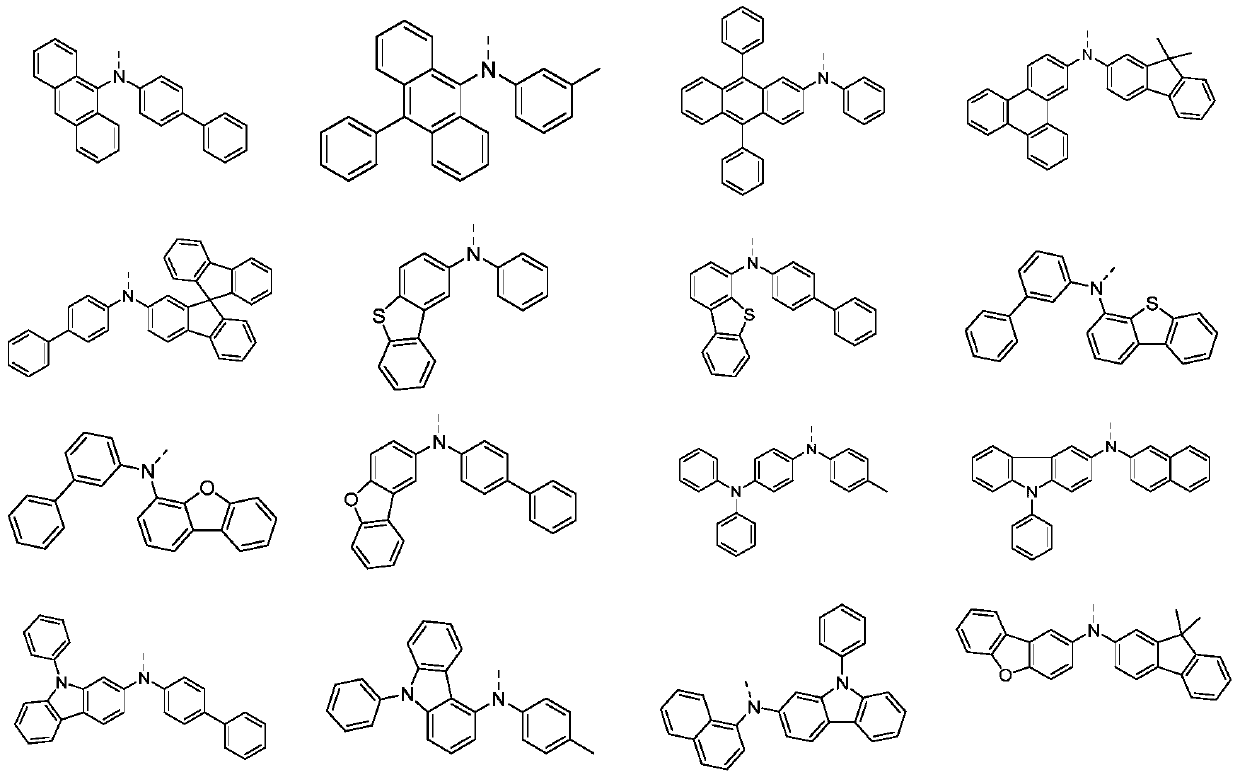
[0051] By adopting the preparation method provided by the invention, the invention provides a series of indenofluoranthene structure compounds.
Embodiment 1
[0053]
[0054] The synthetic route is as follows:
[0055]
[0056] The synthesis of compound I-4 comprises the following specific steps:
[0057] Take a 1-liter three-neck flask, equipped with magnetic stirring, and add potassium tert-butoxide (36.2g, 0.376mol), 3,4'-dimethyldiphenylamine (41.37g, 0.21mol, purity 99%) and toluene in sequence after nitrogen replacement 100ml. After nitrogen replacement again, (1.2 g, 0.006 mol) tri-tert-butylphosphine and (0.7 g, 0.003 mol) palladium acetate were added in sequence. After the addition was complete, the temperature was raised to 85°C. A solution consisting of (43.41 g, 0.1 mol, purity 99%) M1 and 100 ml of toluene was started to be added dropwise, and the temperature was controlled to react within the range of 80-120° C. for 4 hours, and the reaction was completed. Adjust to neutrality, separate the organic phase, extract, dry, perform column chromatography, and spin dry the solvent to obtain 53.3 g of a light yellow s...
Embodiment 2
[0060]
[0061] The synthetic route is as follows:
[0062]
[0063] The synthesis of compound 1-5 comprises the following specific steps:
[0064] N-(1,1'-biphenyl)-3-yl)naphthalene-1-amine and M2 are used to replace 3,4'-dimethyldiphenylamine and M1 described in Example 1 in equal equivalents, and other reactions The conditions and operations were the same as in Example 1, and 74.23 g of a light yellow solid was obtained, with a yield of about 86%.
[0065] Product MS (m / e): 862.33; Elemental analysis (C 66 h 42 N 2 ): theoretical value C: 91.85%, H: 4.91%, N: 3.25%; measured value C: 91.83%, H: 4.92%, N: 3.25%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com