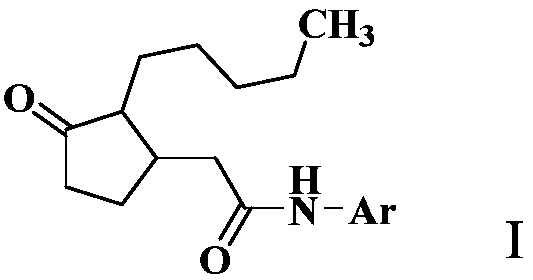
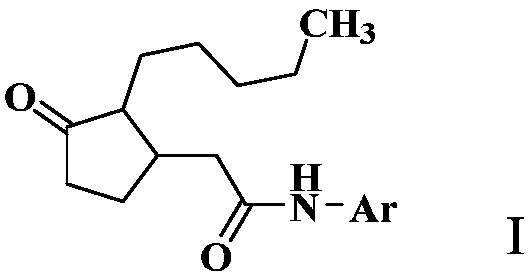
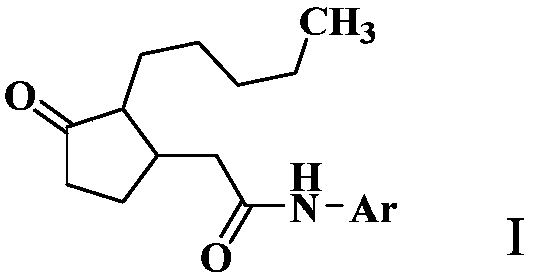
2-(3-oxo-2-pentylcyclopentyl) acetamide derivative and application thereof
A technology of pentylcyclopentyl and acetamide, which is applied in the field of chemistry and can solve the problems of the lack of plant-induced disease resistance activators, which affect the marketing of drugs, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0101] Embodiment 1: the synthesis of compound 1-4
[0102]
[0103] Add 3.0 g of 2-(3-oxo-2-pentylcyclopentyl)acetic acid, 3.0 g of oxalyl chloride, 150 mL of dichloromethane, and three drops of DMF (N,N-dimethylformamide) into a 250 mL flask. The reaction was stirred at room temperature for 12 hours. After the reaction was completed, the solvent was removed under reduced pressure, and 150 mL of ethyl acetate, 1.5 g of p-toluidine, and 2.0 g of triethylamine were added. The reaction was stirred at room temperature for 2 hours. After the reaction was monitored by TLC, wash with 50mL×3 water, dry the organic phase with anhydrous sodium sulfate for 12 hours, precipitate under reduced pressure, and perform column chromatography (eluent is ethyl acetate and petroleum ether (boiling range 60-90°C) , the volume ratio is 1:6) to obtain 2.3 grams of compound 2-(3-oxo-2-pentylcyclopentyl)acetyl 4-methylaniline as a white solid.
Embodiment 2
[0104] Embodiment 2: the synthesis of compound 1-4
[0105]
[0106] In a 250mL flask, add 3.0 grams of 2-(3-oxo-2-pentylcyclopentyl)acetic acid, EDCI (1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride ) 5.0 grams, triethylamine 2.0 g, p-toluidine 1.5 grams, dichloromethane 150 mL. The reaction was stirred at room temperature for 12 hours. After completion of the reaction, wash with 50 mL×3 of water, dry the organic phase with anhydrous sodium sulfate for 12 hours, precipitate under reduced pressure, and perform column chromatography (eluent is ethyl acetate and petroleum ether (boiling range 60-90° C.), volume The ratio is 1:6) to obtain 1.7 g of the compound 2-(3-oxo-2-pentylcyclopentyl)acetyl-p-toluidine as a white solid.
[0107] Other compounds shown in formula I can also be obtained according to the above synthetic route, and the characterization data of other compounds are as follows:
[0108] Compounds 1-3:
[0109] 1 H NMR (600MHz, Chloroform-d) δ7.62(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com