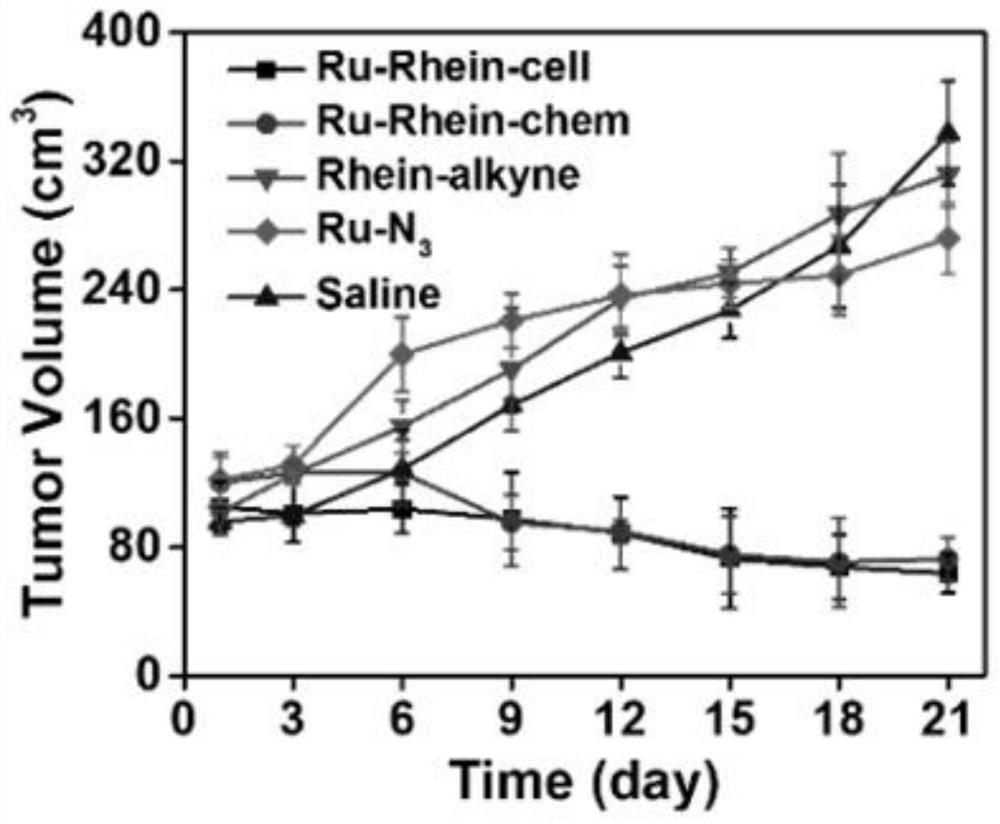
High-efficiency and low-toxicity anticancer compound synthesized by autocatalysis in cells and living bodies and its synthesis method
A synthesis method and compound technology, applied in the direction of steroidal compounds, osmium organic compounds, ruthenium organic compounds, etc., can solve the problems of cell damage, toxic side effects of cancer patients, limited application, etc., and achieve good tumor growth, good anti-tumor effect, Effect with little side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042]
[0043] Under argon protection, 0.5 mmol rhein, 0.5 mmol 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride, 0.5 mmol triethylamine and 0.5 mmol 4-dimethylaminopyridine Dissolve in DMF (N,N-dimethylformamide) and stir overnight (reaction temperature is room temperature, stirring time is 10-24h), after the reaction is completed, DMF is removed by rotary evaporation, washed with water, extracted with chloroform, Dry, separate and purify by column chromatography to obtain a yellow powdery product with a yield of 56%. 1 H NMR (400MHz, DMSO, 25°C, TMS) δ11.91(s, 1H), 9.41(t, J=5.5Hz, 1H), 8.15(d, J=1.7Hz, 1H), 7.89-7.80(m , 1H), 7.79-7.72(m, 1H), 7.42(dd, J=8.4, 1.2Hz, 1H), 4.10(dd, J=5.5, 2.5Hz, 1H), 3.35(s, 5H); 13 C NMR (100MHz, DMSO, 25°C, TMS) δ191.85, 181.46, 164.18, 161.83, 161.57, 141.26, 138.04, 134.05, 133.67, 125.01, 122.97, 119.92, 118.18, 118.01, 116.37, 83 40.31, 40.11, 39.90, 39.69, 39.48, 39.27, 29.27. ESI-MS [Rhein-alkyne] + : Theoretical valu...
Embodiment 2
[0047]
[0048] Under argon protection, 0.03mmol of dichloro(p-methylisopropylphenyl)ruthenium (II) dimer and 0.06mmol of 4-methyl-2,2-bipyridine-azide (prepared by reference: Xun , Z.Q.et al.J.Mater.Chem.A, 2015, 3, 12965-12971) was dissolved in methanol solution and reacted at 65°C for 24h; Stir at room temperature for 0.5-24 hours, and finally add diethyl ether to precipitate to obtain a relatively pure yellow powder product with a yield of 43%, named Ru-N 3 . 1 H NMR (400 MHz, CD 3 OD, 25°C, TMS) δ9.43(d, J=5.9Hz, 1H), 9.29(d, J=5.8Hz, 1H), 8.45(d, J=19.1Hz, 2H), 7.68(ddd, J =44.9, 5.9, 1.4Hz, 2H), 6.15-6.05(m, 2H), 5.88-5.76(m, 2H), 4.83(s, 2H), 2.65(m, 4H), 2.28(s, 3H), 1.06 (dd, J = 6.9, 1.0 Hz, 6H). ESI-MS (+): theoretical value: [Ru-N 3 -PF 6 ] + : Theoretical value: m / z: 496.08, experimental value: m / z 496.25.
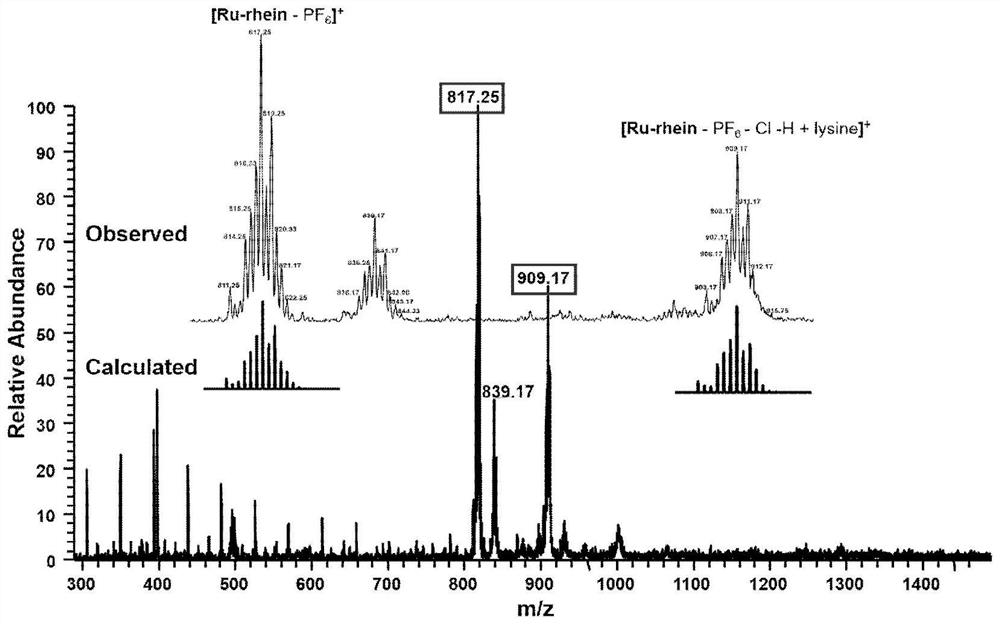
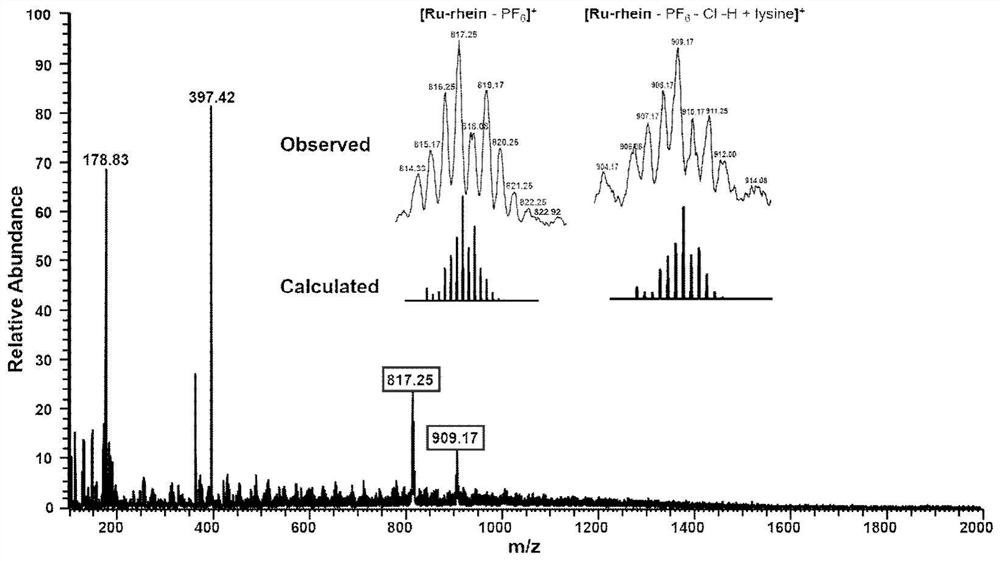
[0049] 0.05mmol Rhein-alkyne prepared in Example 1, 0.05mmol Ru-N 3 and 0.01 mmol of copper sulfate and 0.01 mmol of sodium ascorbate were dissolv...
Embodiment 3
[0053]
[0054] Under the protection of argon, 0.05 mmol of dichloro(p-methylisopropylphenyl) osmium (II) dimer and 0.1 mmol of 4-methyl-2,2-bipyridine-azide were dissolved in methanol, 70 React at ℃ for 48 hours, add 20 times the equivalent of ammonium hexafluorophosphate to it after the reaction, stir at room temperature for 0.5-24 hours, and finally add ether to precipitate to obtain a relatively pure yellow powder product with a yield of 32%, named Os -N 3 . 1 H NMR (400MHz, CD 3 OD, 25°C, TMS) δ9.21(d, J=5.9Hz, 1H), 9.02(d, J=5.8Hz, 1H), 8.33(d, J=19.1Hz, 2H), 7.52(ddd, J =44.9, 5.9, 1.4Hz, 2H), 6.31-6.15(m, 2H), 5.74-5.59(m, 2H), 4.81(s, 2H), 2.58(m, 4H), 2.23(s, 3H), 1.11 (dd, J = 6.9, 1.0 Hz, 6H). ESI-MS (+): theoretical value: [Os-N 3 -PF 6 ] + : Theoretical value: m / z: 586.14, experimental value: m / z 586.35.
[0055] 0.06mmol Rhein-alkyne prepared in Example 1, 0.06mmol Os-N 3 and 0.012 mmol of cuprous iodide were dissolved in DMF and stirred for 12 h. Aft...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com