Preparation method of isocoumarin derivative
A technology for isocoumarin and derivatives, which is applied in the field of preparation of isocoumarin derivatives, can solve the problems of single reaction mode and less fluorine-containing isocoumarins, etc., and achieves the effects of simple operation, diverse types and rich development.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
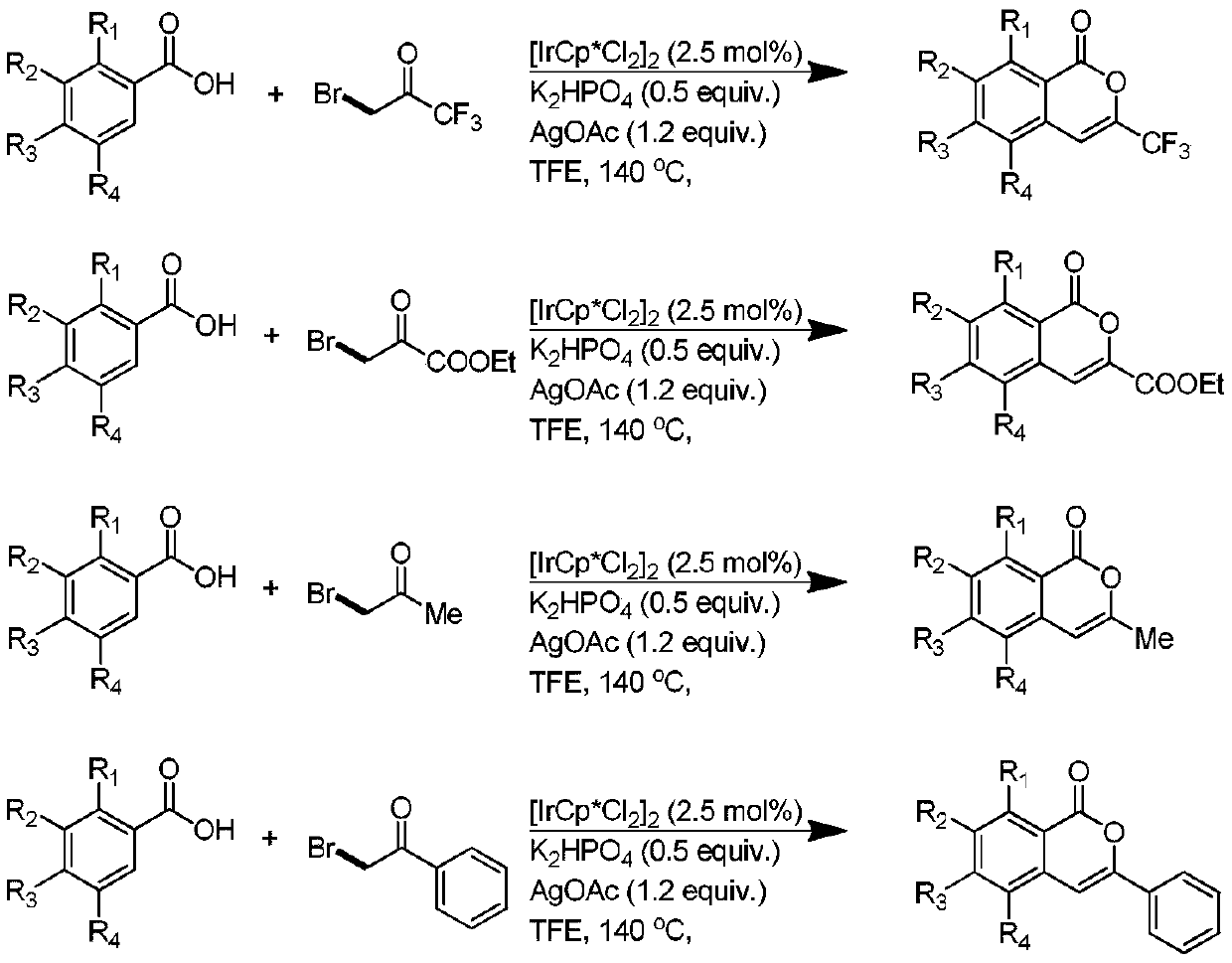
[0033] The invention provides a preparation method of isocoumarin derivatives, comprising: sequentially adding benzoic acid derivatives, pentamethylcyclopentadiene iridium dichloride dimer, dipotassium hydrogen phosphate, and silver acetate into a glass reaction tube and bromotrifluoroacetone, ethyl bromopyruvate, bromoacetophenone, or bromoacetone, etc., put into a stirring bar, use trifluoroethanol as a solvent, and fix the glass reaction tube in a heating stirrer for Stirring, after the reaction is completed, the product is subjected to column chromatography separation and purification treatment to obtain isocoumarin derivatives.
[0034] The reaction process of above-mentioned technical scheme can be expressed as:
[0035]
Embodiment 1
[0039] This implementation case shows a preparation method of isocoumarin derivatives according to the following steps: using 3-methylbenzoic acid as a raw material, the reaction formula is as follows:
[0040]
[0041] (1) Add 0.0326 grams (0.24 mmol) of m-toluic acid, 0.0040 grams (0.005 mmol) of pentamethylcyclopentadiene iridium dichloride dimer, 0.0174 grams (0.1 mmol) of dipotassium hydrogen phosphate, 0.040 g (0.24 mmol) of silver acetate, 0.0382 g (0.2 mmol) of bromotrifluoroacetone and 1 mL of trifluoroethanol were reacted at 140°C for 24 hours;
[0042] ⑵ TLC tracking reaction until the complete end;
[0043] (3) The crude product obtained after the reaction finishes is separated by column chromatography (petroleum ether: ethyl acetate=30:1),
[0044] The target product was obtained (yield 71%).
[0045] 1 H NMR (400MHz, CDCl 3 )δ8.11(s,1H),7.62(dd,J=7.9,1.3Hz,1H),7.45(d,J=8.0Hz,1H),6.95(s,1H),2.50(s,3H). 13 C NMR (101MHz, CDCl 3 )δ160.0,141.7(q,J C-F =58...
Embodiment 2
[0047] This implementation case shows a kind of preparation method of isocoumarin derivative according to the following steps: using 2-chlorobenzoic acid as raw material, its reaction formula is as follows:
[0048]
[0049] (1) Add 0.0374 grams (0.24 mmol) of 2-chlorobenzoic acid, 0.0040 grams (0.005 mmol) of pentamethylcyclopentadiene iridium dichloride dimer, 0.0174 grams (0.1 mmol) of dipotassium hydrogen phosphate, 0.040 g (0.24 mmol) of silver acetate, 0.0382 g (0.2 mmol) of bromotrifluoroacetone and 1 mL of trifluoroethanol were reacted at 140°C for 24 hours;
[0050] ⑵ TLC tracking reaction until the complete end;
[0051] (3) The crude product obtained after the reaction was separated by column chromatography (petroleum ether:ethyl acetate=30:1) to obtain the target product (yield 61%).
[0052] 1 H NMR (400MHz, CDCl 3 )δ7.70–7.66(m,2H),7.48–7.43(m,1H),6.93(s,1H). 13 CNMR (101MHz, CDCl 3 )δ156.2,143.2(q,J C-F =39.4Hz), 138.2, 137.3, 135.4, 133.7, 126.4, 118.4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com