Preparation and application of sulfanilamide and benzothiazole compounds containing tetrahydroisoquinoline
A technology of tetrahydroisoquinoline and benzothiazole, which is applied in the fields of medicinal chemistry and pharmacotherapeutics, can solve the problems of lack of in vivo activity and low efficacy, and achieve the effect of easy-to-obtain raw materials and simple and easy preparation methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
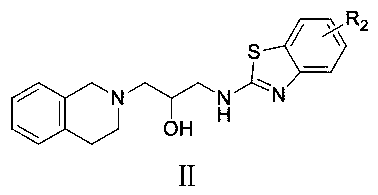
[0025] Example 1: Rational design of compounds A_1~A_10 and B_1~B_2.
[0026] Since GSK3326595 and EPZ015666 were the best two of the previously reported substrate binding site inhibitors, we selected them as template compounds for further structural optimization; analysis of the crystal structure data revealed that the N-(3-(3 , 4-dihydroisoquinoline-2(1H))-2-hydroxypropyl) amino fragment interacts with many residues (L319, T323, F327, E435, L437, E444, W579), hydroxyl in PZ015666 It forms stable hydrogen bonds with L437, E444, and W579, indicating that it is a pharmacophore, and this hydroxyl group is retained in the subsequent molecular design. Therefore, N-(3-(3,4-dihydroisoquinoline -2(1H))-2-hydroxypropyl)amino, replace the left part of EPZ015666 with a group that can form a hydrophobic interaction with PRMT5; according to this design strategy, using traditional structure optimization methods, compounds A_1~A_10 and B_1~B_2, the structure is designed as Image 6 shown....
Embodiment 2
[0027] Embodiment 2: the preparation of compound A_1~A_10.
[0028] 1,2,3,4-Tetrahydroisoquinoline (compound 1) reacts with 2-(chloromethyl)oxirane under basic conditions to obtain the intermediate 2-(oxocyclo-2-ethylmethyl )-1,2,3,4-tetrahydroisoquinoline (compound 2); benzenesulfonyl chloride derivative (compound 3) was treated with ammonium hydroxide to obtain benzenesulfonamide derivative (compound 4); in solid-liquid phase transfer Under catalytic conditions, compound 2 reacts with compound 4 to obtain A_1~A_10 through ring opening; the preparation route is as follows Figure 4 as shown, Figure 4 Middle (a): 2-(chloromethyl)oxirane, potassium carbonate, methanol, room temperature; (b): ammonium hydroxide, acetonitrile, 0°C; (c) potassium carbonate, benzyltriethyl chloride Ammonium chloride, 1,4-dioxane, 90°C.
[0029] N-(3-(3,4-dihydroisoquinolin-2(1H)-yl)-2-hydroxypropyl)benzenesulfonamide (A_1): yellow oil (130mg, 75%); 1 H-NMR (600 MHz, CDCl 3 ) δ 7.89-7.83 (m, 2...
Embodiment 3
[0039] Embodiment 3: Preparation of compounds B_1~B_2.
[0040] Treatment of 2-chloro-6-nitrobenzimidazole (compound 5) with tetrahydro-2H-pyran-4-amine gave 6-nitro-N-(tetrahydro-2H-pyran-4-yl)benzene And[d]thiazol-2-amine (compound 6) or 1-(3,4-dihydroisoquinolin-2(1H)-yl-3-((6-nitrobenzimidazol-2-yl) Amino) propan-2-alcohol (compound 7), reducing compound 6 to obtain an amine intermediate, and the amine intermediate reacts with compound 2 to obtain compound B_1; after reducing compound 7, reductive amination obtains B_2. The preparation route is as follows Figure 5 as shown, Figure 5 Middle (a): Tetrahydro-2H-pyran-4-amine or 1-amino-3-(3,4-dihydroisoquinolin-2(1H)-yl)propan-2-ol, potassium carbonate, N,N-Dimethylformamide, 115 °C; (b): i) palladium / carbon, hydrogen, methanol, ii) 2, ethanol, 120 °C, sealed; (c) i) palladium / carbon, hydrogen , methanol, ii) sodium cyanoborohydride, dihydro-2H-pyran-4(3H)-one, methanol.
[0041] 1-(3,4-Dihydroisoquinolin-2(1H)-yl)-3-((...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap