Acid removal method of lithium salt, non-aqueous electrolyte and battery
A technology of non-aqueous electrolyte and lithium salt, which is applied in the field of batteries containing the non-aqueous electrolyte, and can solve the problem of high free acid content of lithium salt
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0087] Weigh 20g of the crude LiBOB prepared by the liquid phase method, and through testing its free acid content is 6232ppm, the free acid content contained in it is calculated to be about 0.15g. Dissolve 20 g of LiBOB sample in 200 mL of acetone, stir and dissolve at 60 °C to obtain a solution. Then add 2g of KS-6 and 5g of lithium carbonate powder into the solution, and continue to stir for 12h. After heating and stirring, it was cooled to room temperature, and the filter residue was filtered off. Afterwards, the filtrate was rotary evaporated to saturation, 100 mL of toluene was added and stirred again for 6 h until a large amount of LiBOB powder was precipitated, and LiBOB after acid removal was obtained after filtration. The LiBOB after acid removal was tested for free acid, the free acid content was 487ppm, and the acid removal rate was 92.2%.
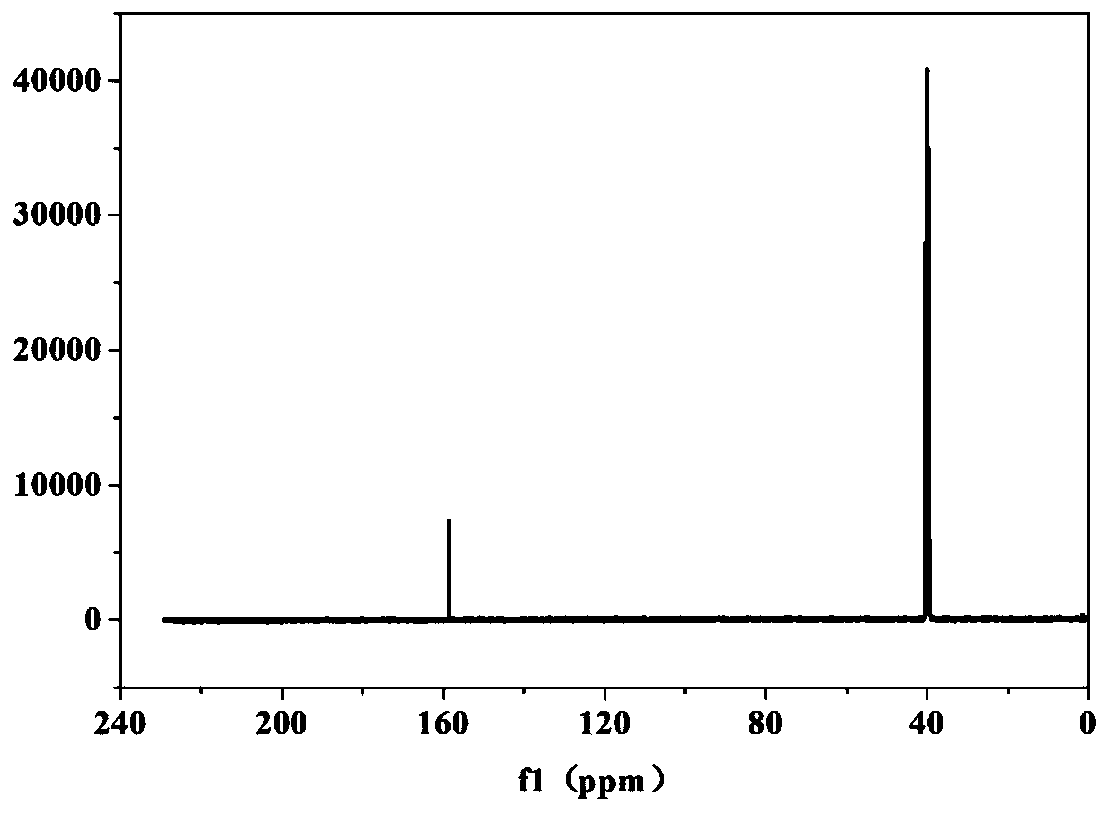
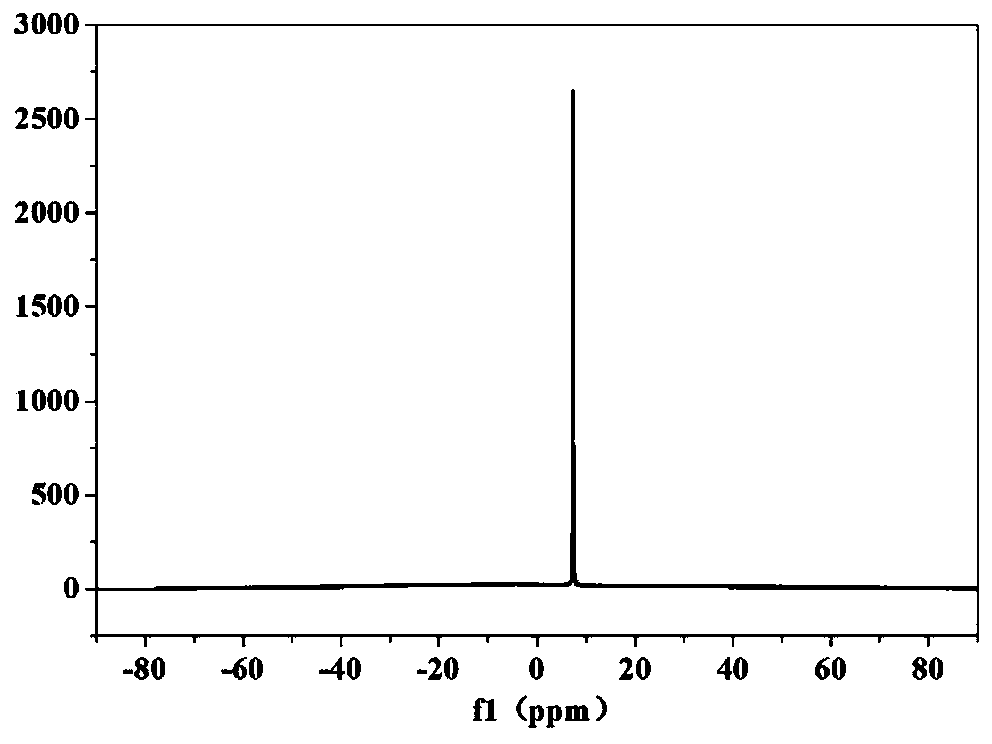
[0088] The obtained LiBOB after deacidification is carried out nuclear magnetic spectrum C spectrum and B spectrum test, an...
Embodiment 2
[0090] Weighed 30g of crude LiBOB prepared in solid phase, and tested that its free acid content was 6550ppm, and calculated that the free acid content contained in it was about 0.2g. Dissolve 30g of LiBOB sample in 350mL of tetrahydrofuran, stir and dissolve at 80°C to obtain a solution. Afterwards, 2.5 g of graphite and 10 g of sodium hydroxide powder were added to the solution, and stirring was continued for 8 h. After heating and stirring, it was cooled to room temperature, and the filter residue was filtered off. Afterwards, the filtrate was rotary evaporated to saturation, 200 mL of diethyl ether was added and stirred for 4 h again until a large amount of LiBOB powder was precipitated, and LiBOB after acid removal was obtained after filtration. The LiBOB after acid removal was tested for free acid, the free acid content was 617ppm, and the acid removal rate was 90.6%.
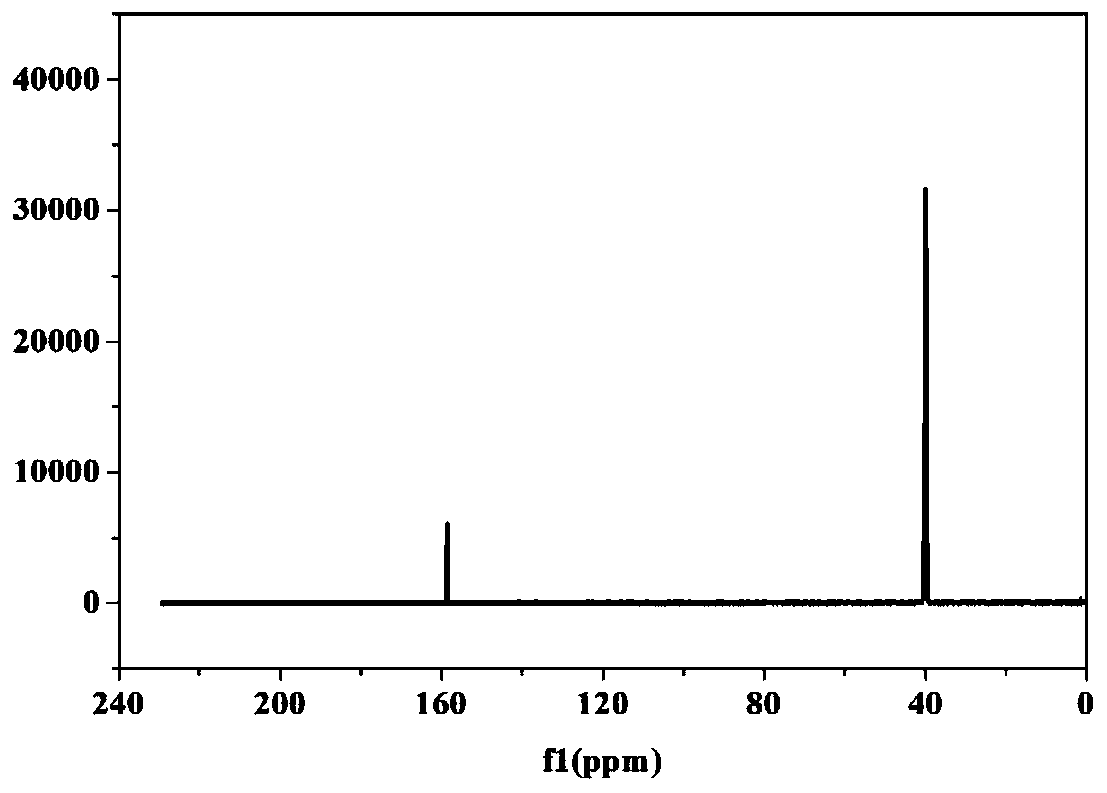
[0091] The obtained LiBOB after deacidification is carried out nuclear magnetic spectrum C spectrum ...
Embodiment 3
[0093] Weigh 50g of crude LiBOB prepared in solid phase, and measure the free acid content of 3948ppm, and calculate the free acid content contained in it to be about 0.2g. Dissolve 50g of LiBOB sample in 700mL of tetrahydrofuran, stir and dissolve at 60°C to obtain a solution. Afterwards, 2 g of carbon nanotubes and 1.5 g of triethylamine were added to the solution, and the stirring was continued for 6 h. After heating and stirring, it was cooled to room temperature, and the filter residue was filtered off. Afterwards, the filtrate was rotary evaporated to saturation, 300 mL of xylene was added and stirred again for 8 h until a large amount of LiBOB powder was precipitated, and LiBOB after acid removal was obtained after filtration. The LiBOB after acid removal was tested for free acid, the free acid content was 717ppm, and the acid removal rate was 81.8%.
[0094] The obtained LiBOB after deacidification is carried out nuclear magnetic spectrum C spectrum and B spectrum te...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com