A preparation method and kit for clinical-grade human induced pluripotent stem cell-derived mesenchymal stem cells
A technology of pluripotent stem cells and mesenchymal stem cells, which is applied in the field of preparation of clinical-grade human induced pluripotent stem cell-derived mesenchymal stem cells, can solve the problems that mesenchymal stem cells cannot be used, and achieve the effect of unified source
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
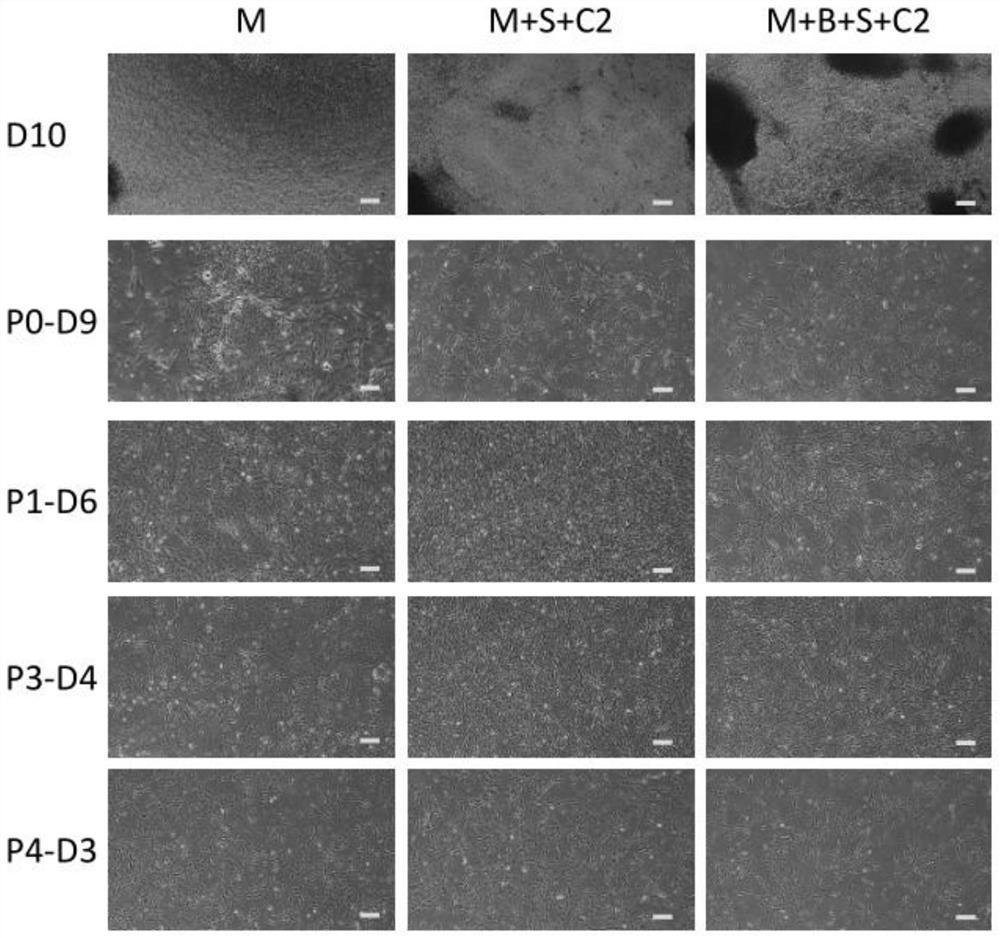
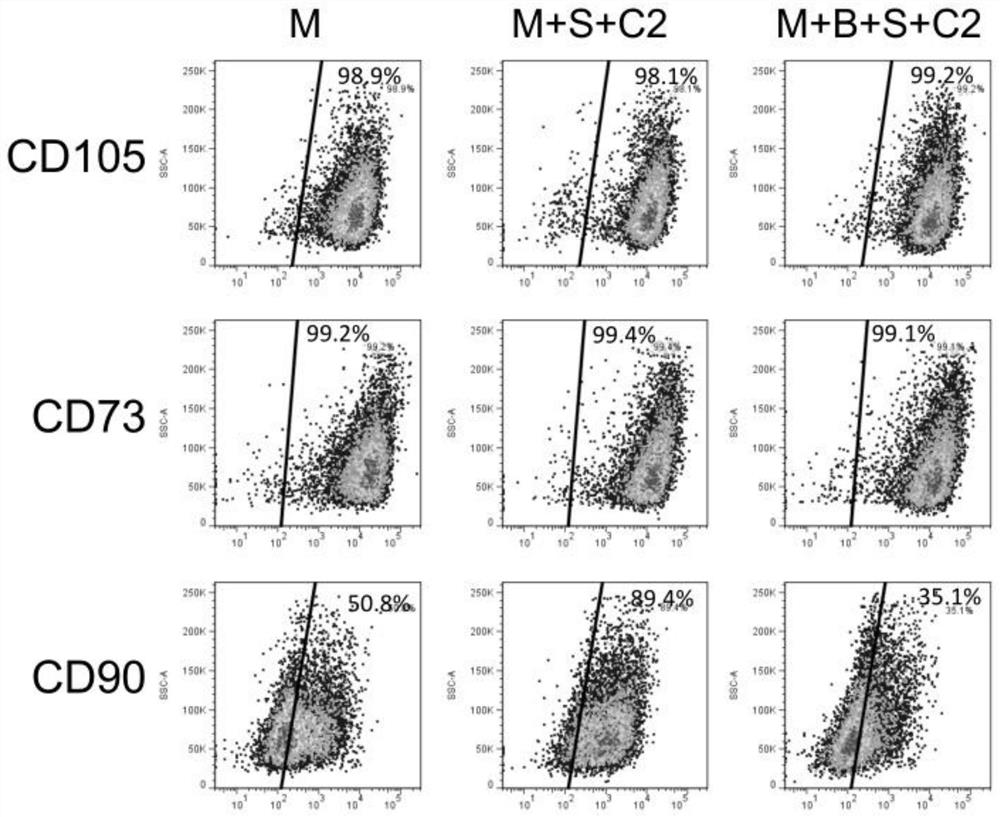
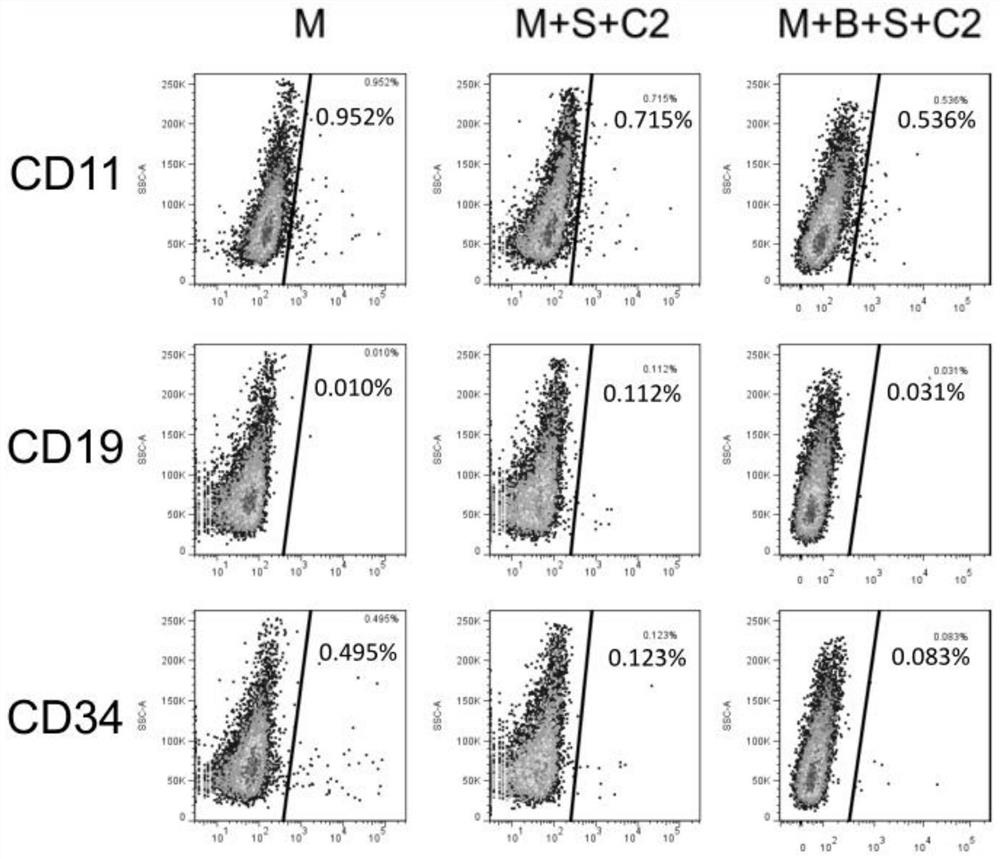
[0034] The invention provides a method for preparing clinical-grade human induced pluripotent stem cell-derived mesenchymal stem cells, comprising the following steps:
[0035]1) digesting human induced pluripotent stem cells to make a single cell suspension, and inoculating it on a pretreated culture plate;
[0036] 2) When the cells reach 80% confluence, use the induction medium for induction culture, and change the medium every day;
[0037] 3) When the cells reach 100% confluence, they are digested to make a single cell suspension, inoculated on the expansion medium for expansion culture, and the cells obtained after 3 to 4 generations of expansion culture are clinical-grade Mesenchymal stem cells.
[0038] In the present invention, human induced pluripotent stem cells are digested to make a single cell suspension, which is seeded on a pretreated culture plate.
[0039] In the present invention, the preparation method of human induced pluripotent stem cells is to separat...
Embodiment 1
[0049] The formula of MSC induction medium (referred to as M+S+C2):
[0050] 1L α-MEM basal medium contains 10% human platelet extract, 10 μmol / L SB431542 and 2 μmol / L CHIR99021. MSC induction medium was obtained after conventional sterilization.
Embodiment 2
[0052] Recipe of MSC Induction Medium:
[0053] 1L α-MEM basal medium contains 10% human platelet extract, 5 μmol / L SB431542 and 5 μmol / L CHIR99021. MSC induction medium was obtained after conventional sterilization.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com