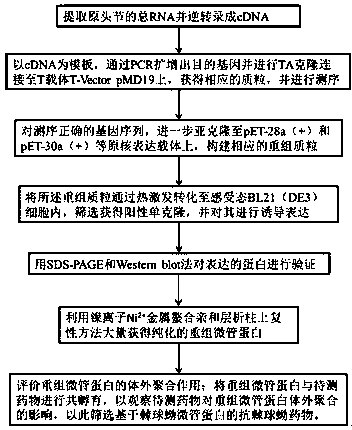
High-throughput anti-echinococcosis drug screening method based on echinococcus microtubulin as targets
A technology for tubulin and echinococcosis, applied in the field of biomedicine, can solve the problems of high research cost, poor absorption, low cure rate, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Embodiment 1: Construction of pET30a-EgTUA9 and pET30a-EgTUB4 plasmid vectors of genetically engineered bacteria (see figure 1 Steps 1, 2, 3)
[0054] (1) Using Echinococcus granulosus cysts from sick sheep in epidemic areas as materials, collect protoscolmets under sterile conditions. The protoscole was stained with 0.1% methylene blue solution, and observed with an inverted microscope, and the protoscole with activity greater than 95% was used for subsequent experiments.
[0055] (2) Use the RNA extraction kit to extract protoscole RNA, and use the cDNA reverse transcription kit to obtain the cDNA of Echinococcus granulosus; using the above cDNA as a template, use the upstream primer CGCGAGCTCATGCGTGAATGTATCAGTAT of EgTUA9 (the underlined part is Sac I Restriction site), the downstream primer AGCGGCCGCTTAGTACTCCTCGCCCTCTT (the underlined part is the restriction site of NotI) amplifies the EgTUA9 fragment (1356bp). PCR reaction conditions: 95°C pre-denaturation for 5...
Embodiment 2
[0060] Embodiment 2: Transformation of genetically engineered bacteria recombinant plasmid and induced expression of protein (see figure 1 Step 4)
[0061] (1) Taking pET30a-EgTUA9 and pET30a-EgTUB4 as examples, describe the transformation of recombinant plasmids of genetically engineered bacteria and the induced expression of proteins. ), incubated on ice for 30min, then heat-shocked at 42°C for 90s, immediately placed on ice and incubated for 3min, then added an appropriate amount of non-resistant LB liquid medium to mix, shaken and cultured at 37°C for 45min, and then applied to 50μg / mL Kanamycin LB solid plate, cultured upside down at 37°C overnight.
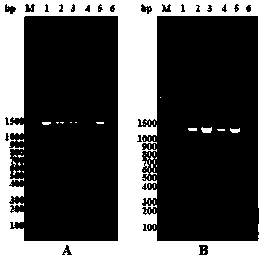
[0062] (2) Pick a single clone and use 5 mL of LB liquid culture containing 50 μg / mL kanamycin to culture overnight at 37°C with shaking; perform colony PCR verification on the single clone respectively, and the results are as follows figure 2 As shown, a band in line with the expected target fragment size appeared; and th...
Embodiment 3
[0064] Embodiment 3: recombinant tubulin expression verification (see figure 1 step 5)
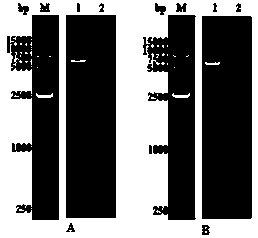
[0065] (1) Before protein induction, take 1 mL of bacterial liquid as the non-induced group, after shaking culture, take 1 mL of bacterial liquid as the induced group, and centrifuge the bacterial liquid of the uninduced group and the induced group to collect the bacterial pellet, add the loading buffer liquid and mix well, set aside. Centrifuge the remaining bacterial liquid to collect the bacterial pellet, resuspend in buffer A (20mM Tris-HCl, 300mM NaCl, 10mM imidazole, pH7.4), and perform ultrasonication on ice, centrifuge at 12000rpm for 20min, and collect The supernatant and the precipitate were dissolved in buffer B (20mM Tris-HCl, 300mM NaCl, 10mM imidazole, 8M urea, pH 7.4), and an appropriate amount of supernatant and precipitation solution were taken respectively and added into an equal volume of loading buffer , respectively marked as supernatant group and precipitation group...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com