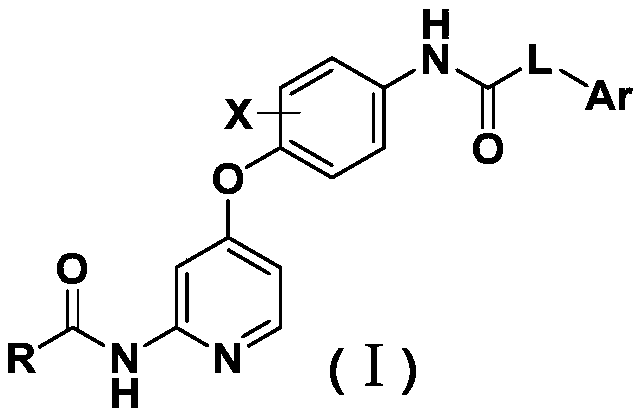
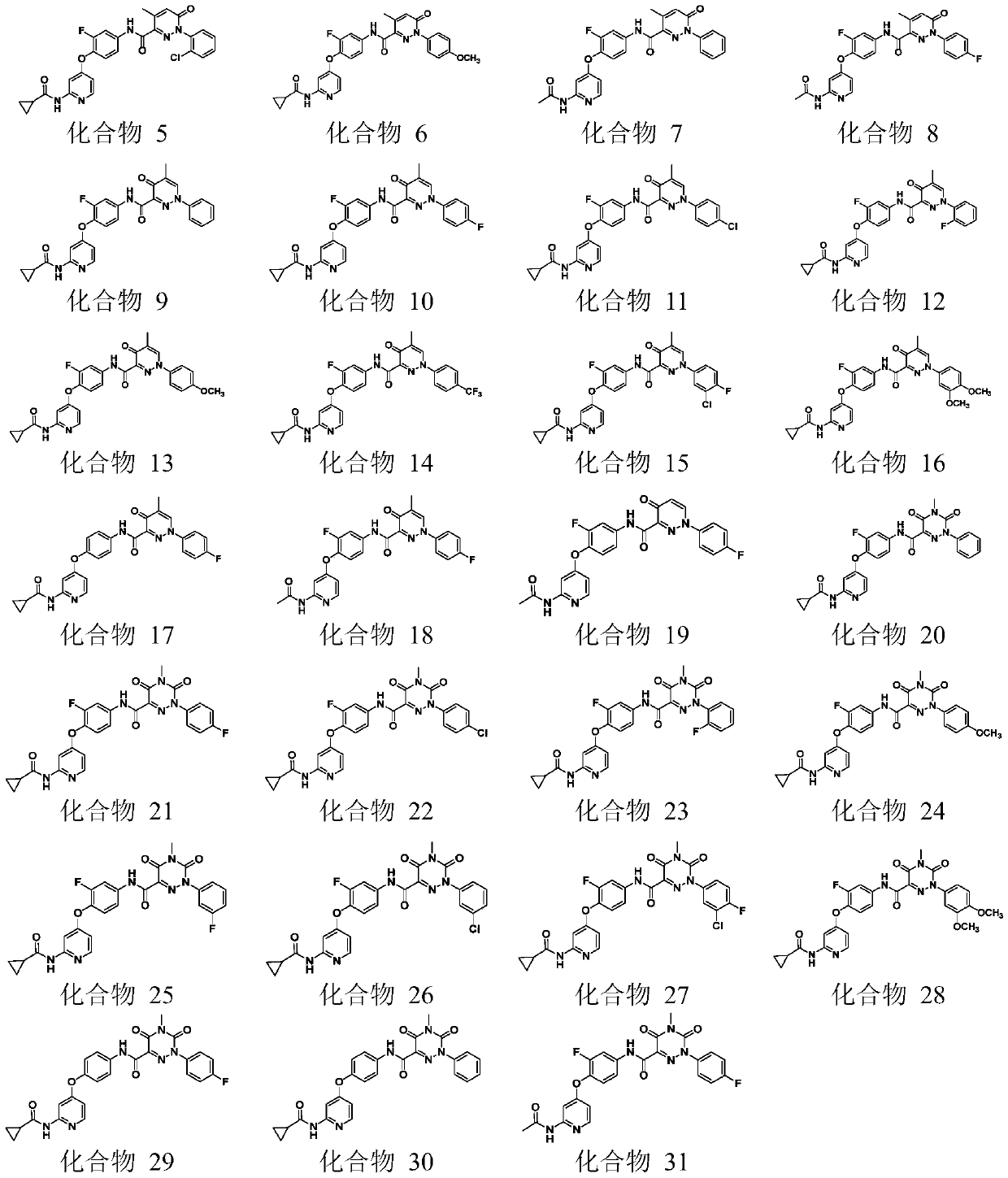
4-phenoxy pyridine derivative containing 3-pyridazinone structure, 4-pyridazinone structure and 1,2,4-triazinone structure, and applications thereof.
A phenoxypyridine and pyridazinone technology, which is applied in the directions of medical preparations containing active ingredients, drug combinations, active ingredients of heterocyclic compounds, etc., can solve the problem of low oral bioavailability, large toxic and side effects, and clinical treatment Problems such as unsatisfactory effects and pharmacokinetic parameters
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
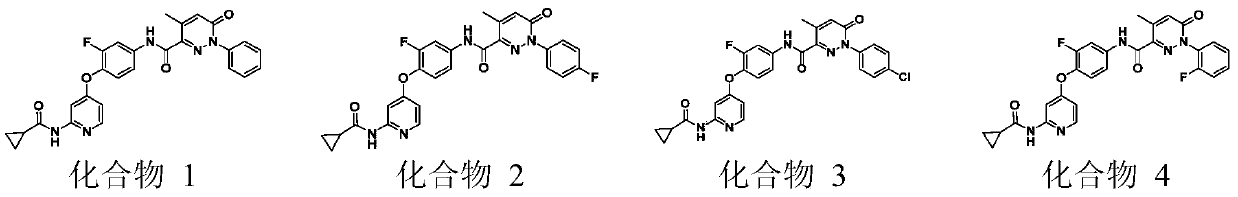
[0062] Example 1: N-(4-{[2-(cyclopropanecarboxamido)pyridin-4-yl]oxy}-3-fluorophenyl)-4-methyl-6-oxo-1-benzene Preparation of 1,6-dihydropyridazine-3-carboxamide (Compound 1)
[0063]
[0064] Step 1 N-(4-chloropyridin-2-yl)cyclopropylformamide (a)
[0065] 8.80g of 2-amino4-chloropyridine and 20.80g of triethylamine were dissolved in 80mL of dichloromethane, and 30mL of dichloromethane solution containing 9.30g of cyclopropylformyl chloride was added dropwise to the solution under ice-bath conditions. Warm up to room temperature after the addition is complete. Stirred for 12h, after the completion of the reaction, the mixture was washed with 20% K 2 CO 3 solution and saturated brine were washed 3 times respectively, the organic phase was separated, dried over anhydrous sodium sulfate, filtered, and the solvent was evaporated to dryness to obtain a crude product, which was separated by column chromatography to obtain a white solid N-(4-chloropyridin-2-yl) Cyclopropylfor...
Embodiment 2
[0079] Example 2: N-(4-{[2-(cyclopropanecarboxamido)pyridin-4-yl]oxy}-3-fluorophenyl)-4-methyl-6-oxo-1-( 4-fluorophenyl)-1,6-dihydropyridazine-3-carboxamide (Compound 2)
[0080]
[0081] 1 H NMR (600MHz, CDCl 3 )δ8.92(s,1H),8.64(s,1H),8.12(d,J=5.7Hz,1H),7.82–7.68(m,2H),7.60(dd,J=8.8,4.7Hz,2H ),7.26–7.18(m,3H),7.14(t,J=8.6Hz,1H),6.91(s,1H),6.64(dd,J=5.7,2.2Hz,1H),2.66(s,3H) ,1.57–1.45(m,1H),1.11–0.97(m,2H),0.93–0.79(m,2H); MS(ESI)m / z(%):518.1[M+H] + ,540.1[M+Na] + .
Embodiment 3
[0082] Example 3: N-(4-{[2-(cyclopropanecarboxamido)pyridin-4-yl]oxy}-3-fluorophenyl)-4-methyl-6-oxo-1-( 4-Chlorophenyl)-1,6-dihydropyridazine-3-carboxamide (Compound 3)
[0083]
[0084] 1 H NMR (600MHz, CDCl 3 )δ8.89(s,1H),8.44(s,1H),8.12(d,J=5.8Hz,1H),7.82–7.66(m,2H),7.59(d,J=8.8Hz,2H), 7.50(d, J=8.8Hz, 2H), 7.24(s, 1H), 7.15(t, J=8.6Hz, 1H), 6.92(t, J=7.4Hz, 1H), 6.63(m, 1H), 2.66(s,3H),1.55–1.43(m,1H),1.07–1.00(m,2H),0.89–0.81(m,2H); MS(ESI)m / z(%):534.1[M+H ] + ,556.1[M+Na] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com