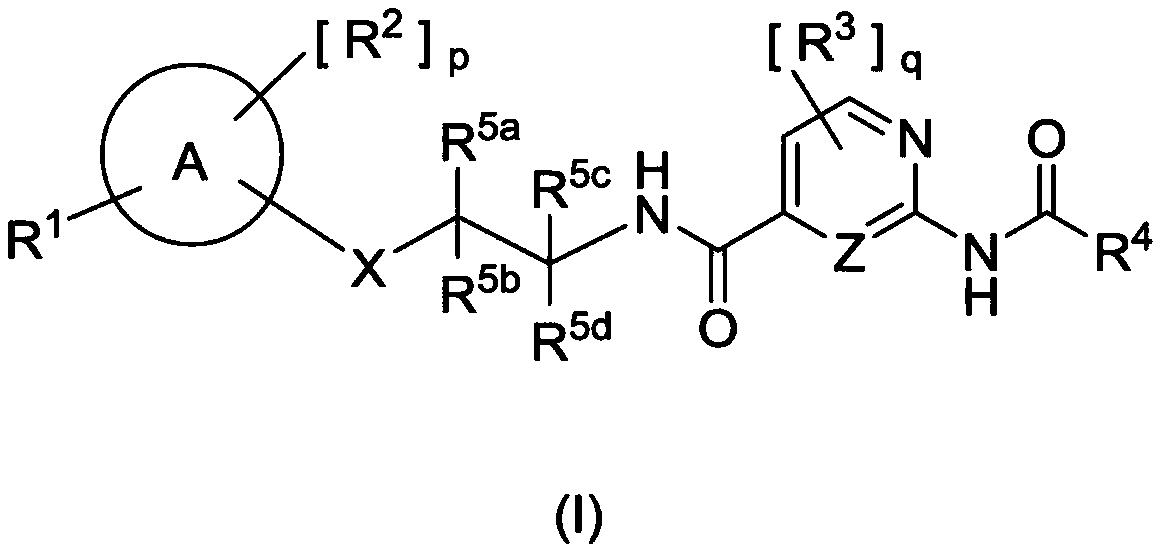
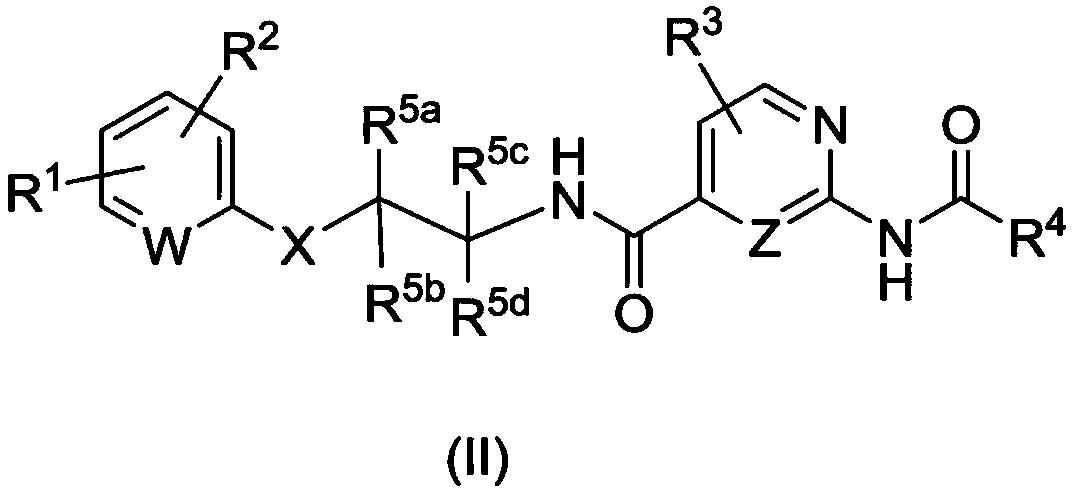
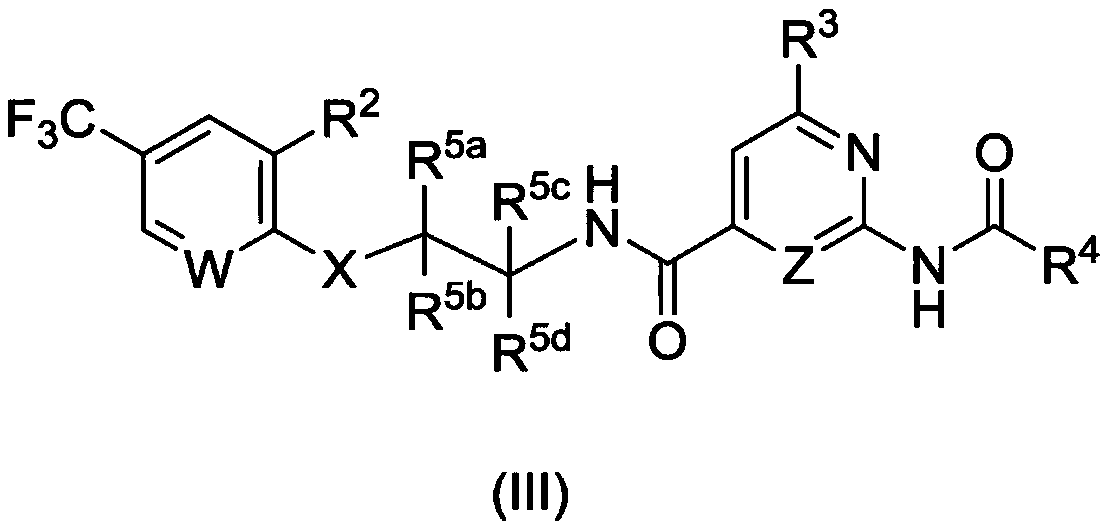
AMIDE DERIVATIVES AS Nav1.7 and Nav1.8 BLOCKERS
一种化合物、烷基的技术,应用在酰胺衍生物领域,能够解决不完整疗效、低效力等问题,达到改善副作用特性的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0940] 2-(Cyclopropanecarboxamido)-N-(2-(4-(trifluoromethyl)phenoxy)propyl)isonicotinamide
[0941] To 2-(4-(trifluoromethyl)phenoxy)propyl-1-amine (15mg, 0.068mmol, amine 1), 2-(cyclopropanecarboxamido)isonicotinic acid (14mg, 0.068mmol, To a mixture of carboxylic acid 2) and N,N-diisopropylethylamine (0.047 mL, 0.27 mmol) in DMF (1 mL) was added HBTU (39 mg, 0.10 mmol) at room temperature. After stirring at 60°C for 2 hours, the mixture was diluted with EtOAc (6 mL), washed with water, and dried over sodium sulfate. The organic layer was purified by NH-silica gel column chromatography and eluted with EtOAc, followed by preparative LC-MS to obtain 8.6 mg of the title compound.
[0942] For the other examples, prepared according to methods similar to those described in Example 1, using appropriate amines and carboxylic acids (see Table 2). The reactants are commercially available materials or can be obtained by conventional methods known to those skilled in the art, otherwis...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com