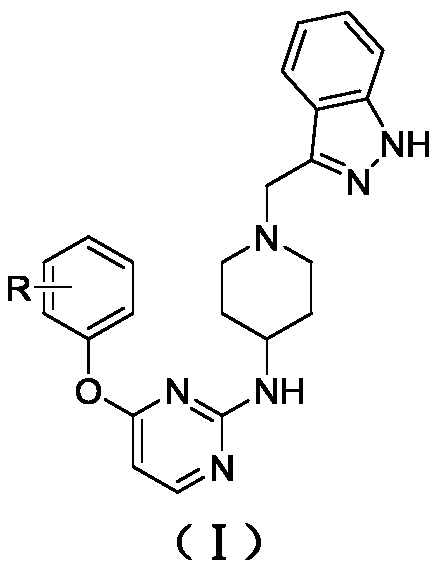
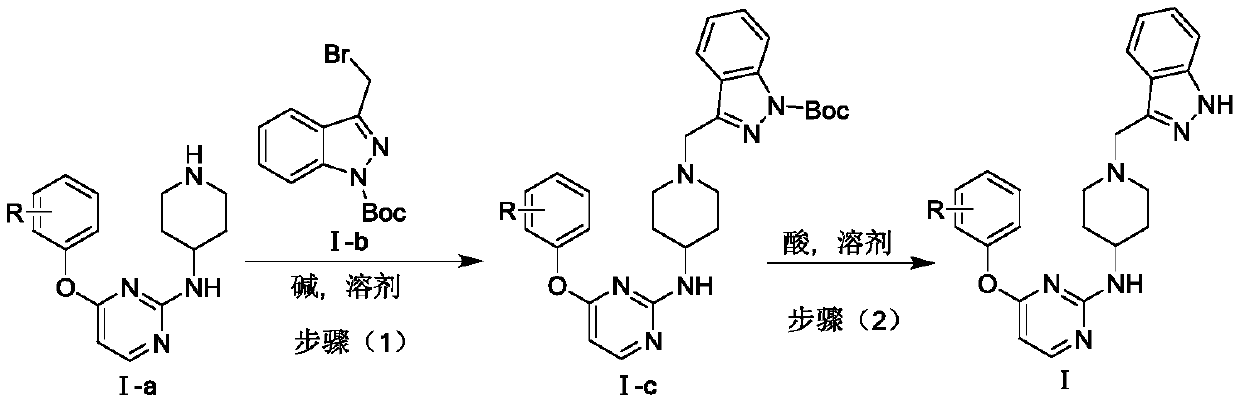
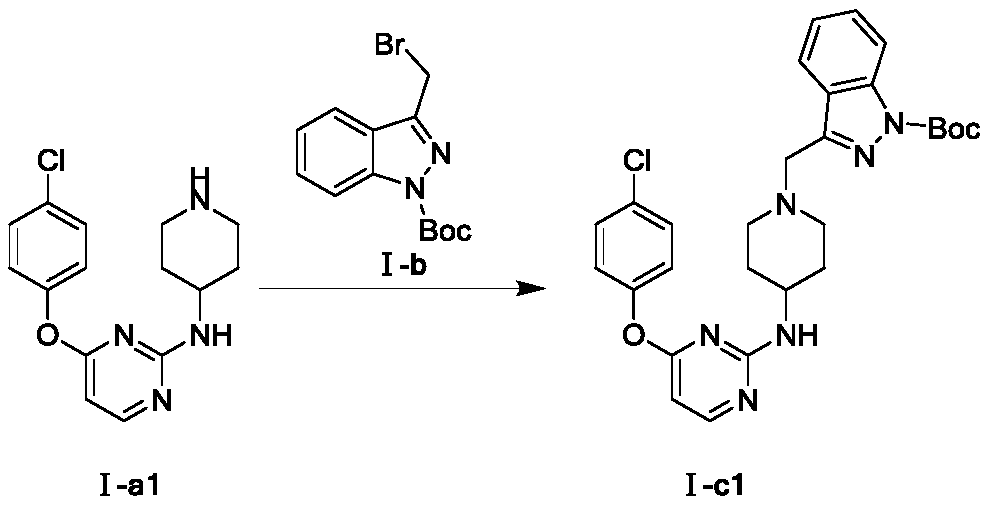
A kind of indazole piperidine pyrimidine derivative and its preparation method and application
A technology of indazole, piperidine pyrimidine and its derivatives, which is applied in the field of medicine, and can solve problems such as severe toxic and side effects, large toxic and side effects, and drug resistance reducing the curative effect of compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1: the synthesis of intermediate Ⅰ-c1
[0026]
[0027] Weigh Ⅰ-a1 (1mmol) and Ⅰ-b (1mmol) and dissolve in 1,4-dioxane (10mL), add K under stirring at room temperature 2 CO 3 , stirred at room temperature for 4h. Thin-layer chromatographic detection showed that the two raw material points had basically reacted completely, so the stirring was stopped. Pour the reaction solution into 50mL water, extract with ethyl acetate (2×50mL), wash with saturated brine (2×40mL), dry the organic phase over anhydrous sodium sulfate, filter with suction, spin the filtrate to obtain an oily substance, and column chromatography I-c1 was purified with a yield of 35.2%.
Embodiment 2
[0028] Embodiment 2: Synthesis of Intermediate I-c1
[0029] Weigh I-a1 (1 mmol) and I-b (1 mmol) and dissolve in N-methylpyrrolidone (10 mL), add DIEA under stirring at room temperature, and stir at room temperature for 4 h. Thin-layer chromatographic detection showed that the two raw material points had basically reacted completely, so the stirring was stopped. Pour the reaction solution into 50 mL of water, extract with ethyl acetate (2×50 mL), wash with saturated brine (2×50 mL), dry the organic phase over anhydrous sodium sulfate, filter with suction, and spin the filtrate to obtain an oily substance, which is subjected to column chromatography I-c1 was purified with a yield of 42.3%.
Embodiment 3
[0030] Embodiment 3: the synthesis of intermediate I-c2
[0031]
[0032] Weigh I-a2 (1 mmol) and I-b (1 mmol) and dissolve in DCM (10 mL), add sodium bicarbonate with stirring at room temperature, and stir at room temperature for 3 h. Thin-layer chromatographic detection showed that the two raw material points had basically reacted completely, so the stirring was stopped. The reaction solution was poured into 50mL water, extracted with DCM (2×50mL), washed with saturated brine (2×50mL), the organic phase was dried over anhydrous sodium sulfate, filtered with suction, and the filtrate was spin-dried to obtain an oil, which was purified by column chromatography to obtain Ⅰ-c2, yield 34.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com