Application of xenicane type diterpenoid compound to preparation of antioxidative neuroprotective drugs
A compound and anti-oxidation technology, which can be used in drug combinations, nervous system diseases, anti-toxins, etc., and can solve the problem of epoxyhydroxyacetyldictyolal having no medicinal function reports, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
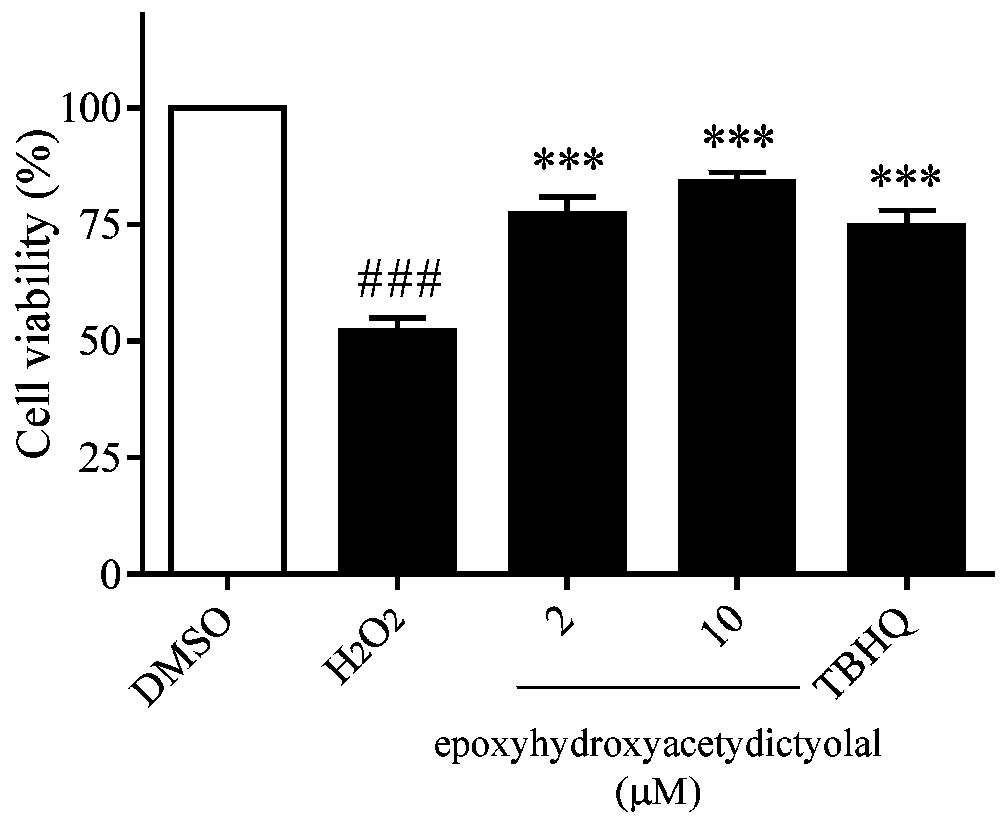
[0026] Example 1 compound to H 2 o 2 Inhibition assay of induced oxidative damage in neuron-like PC12 cells
[0027] The PC12 cells in the logarithmic growth phase were inoculated in a 96-well culture plate at 3000 cells / well, with 100 μL of medium per well, at 37°C with 5% CO 2 overnight in the incubator. Compound (2, 10 μM) or TBHQ (positive control, 10 μM) was added to each well for pre-incubation for 18 h, and then given a certain concentration of H 2 o 2 (450μM) stimulation for 24h, and finally add 20μL of MTT solution (5mg / mL) into the incubator to continue culturing. After 4 hours, the liquid in each well was sucked away, DMSO (120 μL / well) was added, oscillated and mixed for 10 minutes, and the absorbance (A value) was measured at a wavelength of 490 nm with an enzyme-linked immunosorbent assay instrument, and the DMSO group was blank control B. The cell survival rate was calculated (experimental group A value / control group B value×100%), and the experiment was re...
Embodiment 2
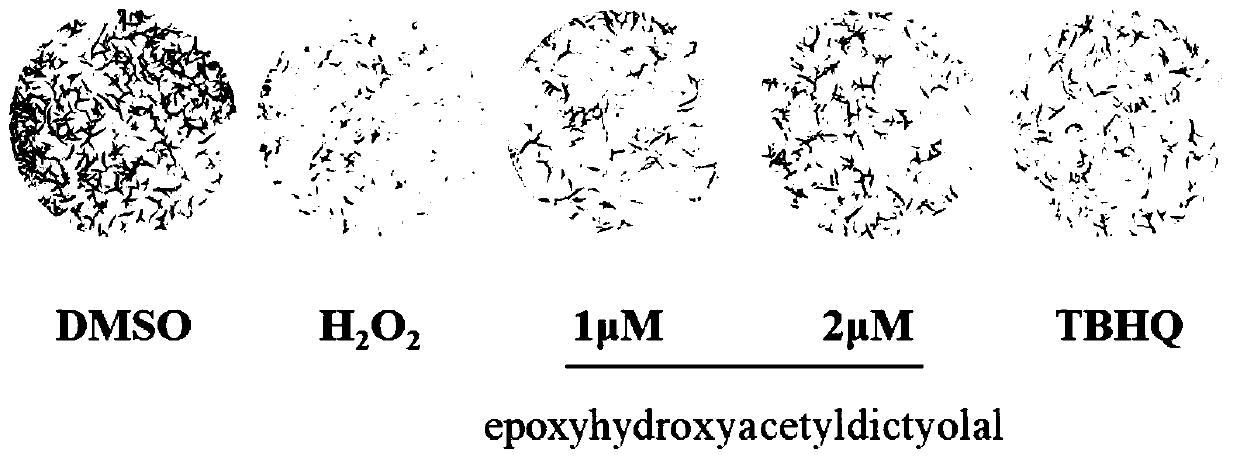
[0029] Example 2 compound to H 2 o 2 Effect of neuron-like PC12 cell colony formation after injury
[0030] PC12 cells in the logarithmic growth phase were seeded in a 12-well culture plate at 1000 cells / well, with 1 mL of medium per well, and incubated overnight in an incubator. Add compounds (1, 2 μM) or TBHQ (positive control, 2 μM) to each well for pre-incubation for 24 hours, and then give a certain concentration of H 2 o 2 (100 μM) stimulation. After 24 hours, replace with fresh medium, place in an incubator containing 5% CO2 at 37°C and continue culturing for 6 days until the cells grow to visible colonies. After fixing with 4% paraformaldehyde, colonies were stained with crystal violet for 15-20 min.
[0031] Experimental results such as figure 2 As shown, colony formation experiments further confirmed the effect of xenicane-type diterpenoid epoxyhydroxyacetyldictyolal on H 2 o 2 Induced protective effect on oxidative damage of PC12 cells, and when the compoun...
Embodiment 3
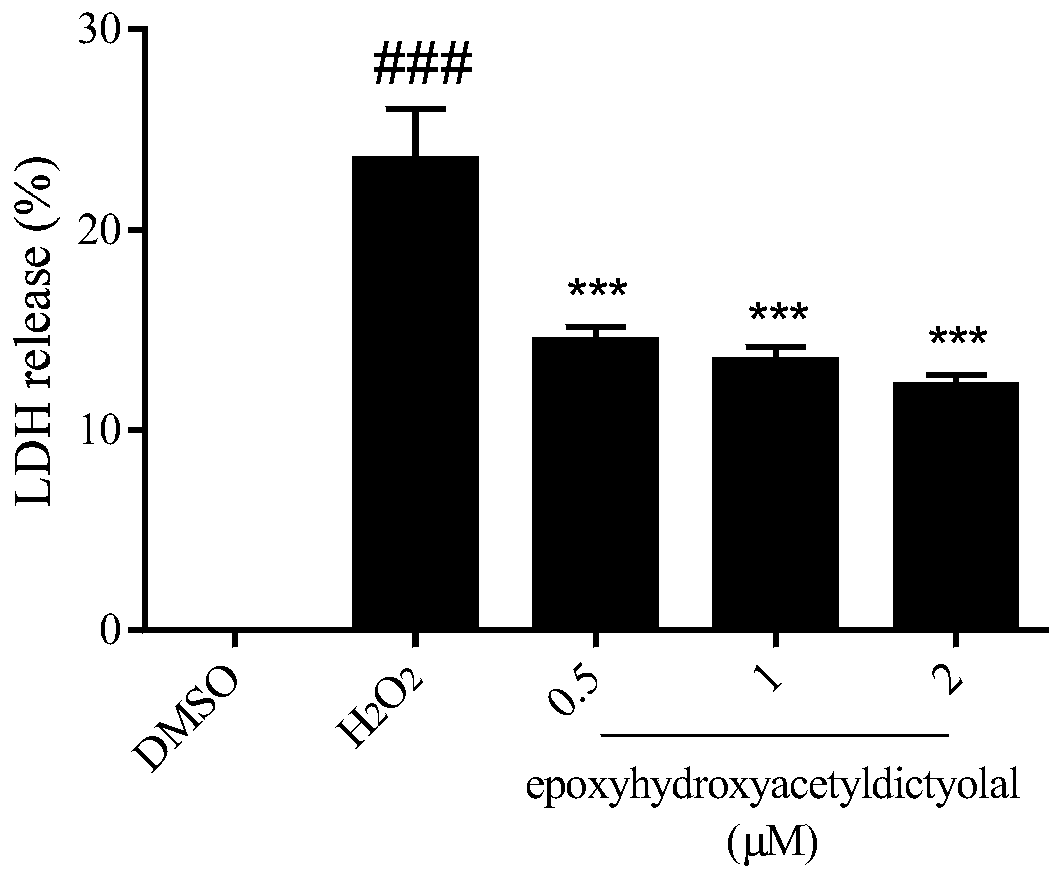
[0032] Example 3 compound to H 2 o 2 Determination of LDH leak rate in induced neuron-like PC12 cells
[0033]Lactate dehydrogenase (LDH) is ubiquitous in every cell and is a common intracellular enzyme. When the cell membrane is damaged, LDH will leak into the culture medium. Therefore, the LDH leakage rate can represent the integrity of the cell membrane and cell viability. The PC12 cells in the logarithmic growth phase were inoculated in a 96-well culture plate at 5000 cells / well, with 100 μL of medium per well, at 37°C containing 5% CO 2 overnight in the incubator. Compounds (0.5, 1, 2 μM) were added to each well for pre-incubation for 18 h, and then given a certain concentration of H 2 o 2 (450μM) stimulation for 24h. After drug stimulation, the leakage rate of LDH was detected according to the instructions of the LDH detection kit.
[0034] Experimental results such as image 3 shown, with H 2 o 2 Compared with the injury group, epoxyhydroxyacetyldictyolal, a xe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com