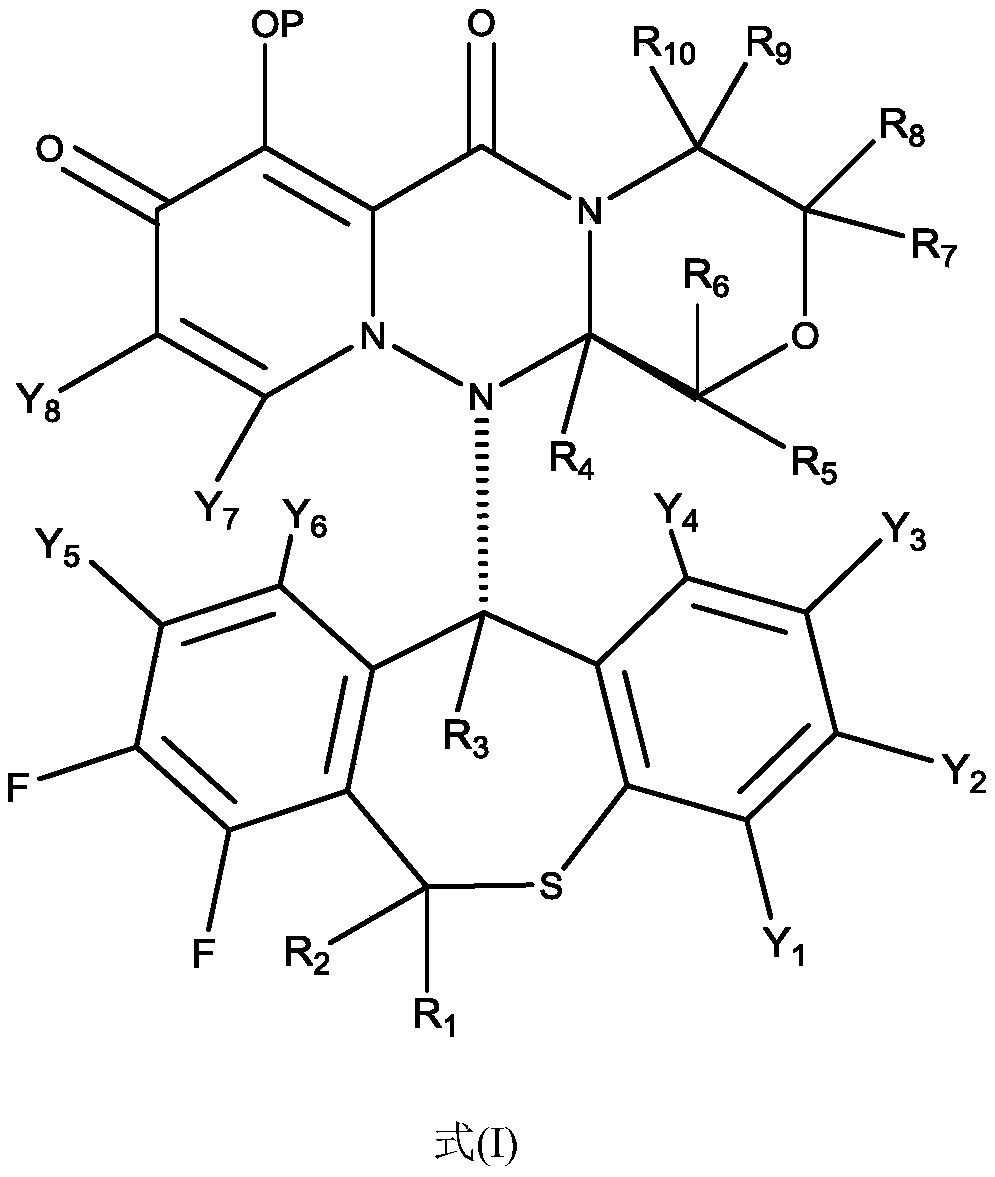
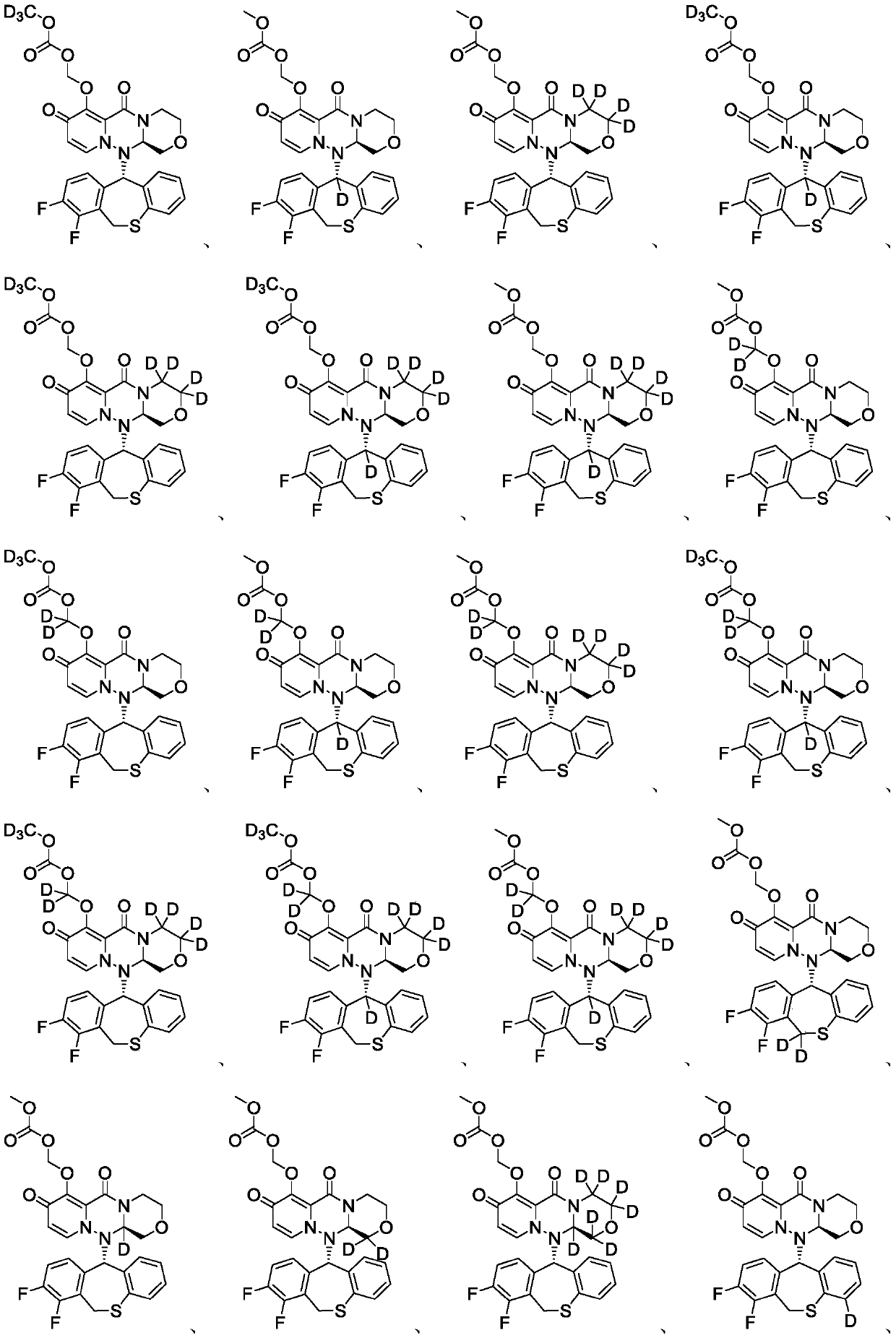
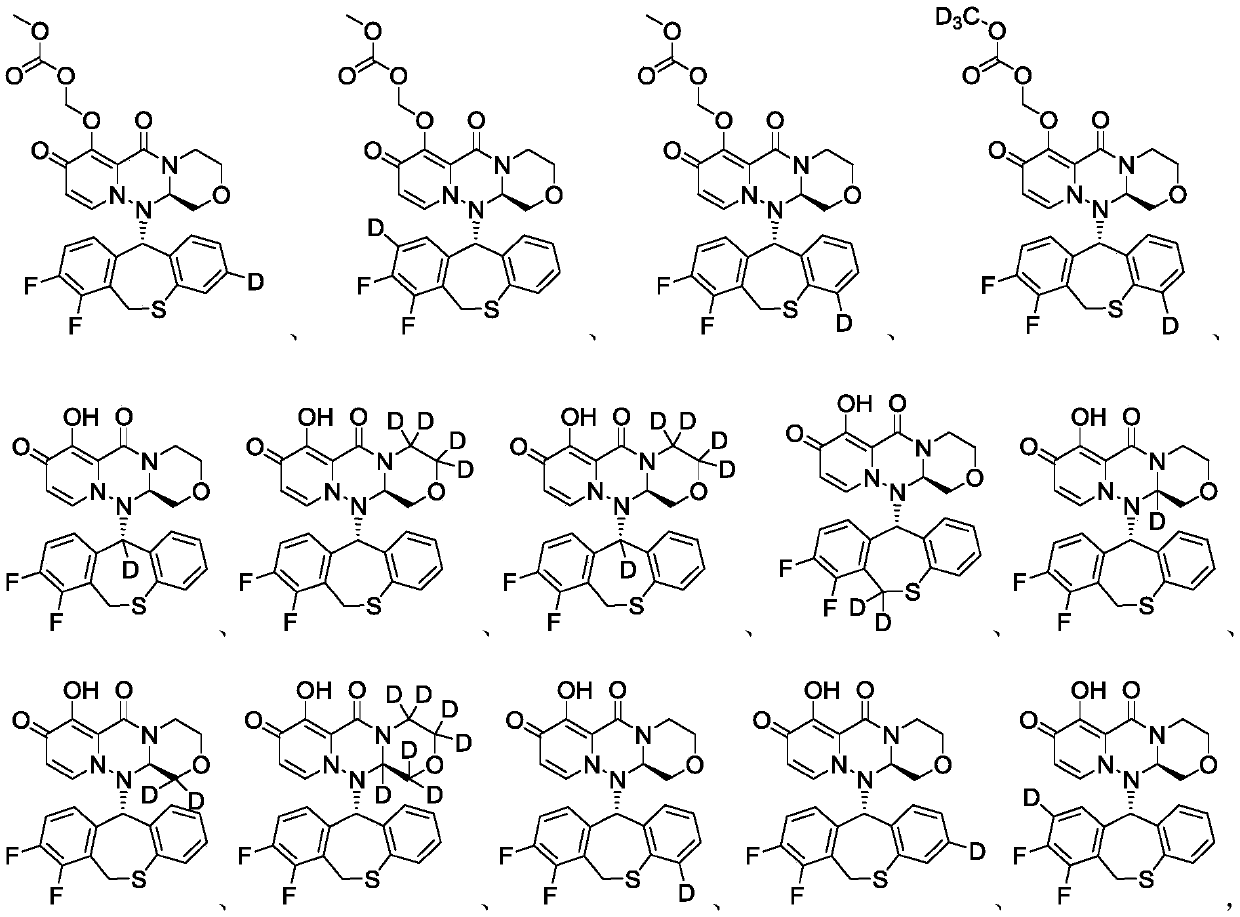
Substituted polycyclic pyridone compound and prodrug thereof
A compound and solvent compound technology, applied in heterocyclic compound isotope introduction, isotope introduction into organic compounds, organic chemistry, etc., can solve problems such as limited application range, poor patient compliance, and unmet clinical needs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0140] The preparation of the compounds of the present invention may involve the protection and deprotection of various chemical groups. The need for protection and deprotection and selection of appropriate protecting groups can be readily determined by those skilled in the art. The chemistry of protecting groups can be found in, eg, Wuts and Greene, Protective Groups in Organic Synthesis, 4th Edition, John Wiley & Sons: New Jersey, (2006), which is incorporated herein by reference in its entirety.
[0141] The compounds of the present invention can be prepared as their individual stereoisomers by reacting a racemic mixture of the compound with an optically active resolving agent to form a pair of diastereoisomeric compounds, separating the diastereomers and recovering the optically pure enantiomers. isomer. Enantiomeric resolution may be performed using diastereomeric derivatives of the compounds of the invention, preferentially dissociable complexes (eg, crystalline diaster...
Embodiment 1
[0224] Example 1 (((12aR)-12-((11S)-7,8-difluoro-6,11-dihydrodibenzo(B,E)thiepin-11-yl- 11-d)-6,8-dioxo-3,4,6,8,12,12a-hexahydro-1H-(1,4)oxazine(3,4-C)pyrido(2,1 -F)(1,2,4) Preparation of triazin-7-yl)oxy)methyl carbonate (compound T-1)
[0225]
[0226] The synthetic route is as follows
[0227]
[0228] Synthesis of step 1 compound 25
[0229] Compound 9 (0.5g, 1.5mmol), compound 24 (0.45g, 1.5mmol) and T3P (0.73g, 2.3mmol) were added to ethyl acetate (6ml) in turn, and then methanesulfonic acid (0.29g , 3.0mmol), the reaction solution was reacted at 70°C for 6 h, then 10ml of water was added, and then extracted with ethyl acetate (20ml×3), the organic phases were combined, dried over anhydrous sodium sulfate, and the concentrated solution was subjected to column separation ( Eluent: petroleum ether / ethyl acetate (v / v)=1:2), 0.6 g of white solid was obtained with a yield of 69%.
[0230] Synthesis of step 2 compound 26
[0231] Compound 25 (0.6g, 1.0mmol) and...
Embodiment 2
[0236] Example 2 (((12aR)-12-((11S)-7,8-difluoro-6,11-dihydrodibenzo(B,E)thiepin-11-yl- 11-d)-6,8-dioxo-3,4,6,8,12,12a-hexahydro-1H-(1,4)oxazine(3,4-C)pyrido(2,1 -F)(1,2,4) Triazin-7-yl-3,3,4,4-d 4 ) Oxygen group) the preparation of methyl carbonate (compound T-2)
[0237]
[0238] The synthetic route is as follows
[0239]
[0240] Synthesis of step 1 compound 28
[0241] Compound 17 (0.5g, 1.5mmol), compound 24 (0.45g, 1.5mmol) and T3P (0.73g, 2.3mmol) were added to ethyl acetate (6ml) solution in turn, and then methanesulfonic acid (0.29g , 3.0mmol), the reaction solution was reacted at 70°C for 6h, then 10ml of water was added, and then extracted with ethyl acetate (20ml×3), the organic phases were combined, dried over anhydrous sodium sulfate, and the concentrated solution was subjected to column separation (washing Removing agent: petroleum ether / ethyl acetate (v / v)=1:2), 0.55 g of white solid was obtained, yield 60%.
[0242] Synthesis of step 2 compound...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com