Construction and application of a hunov GII.4 clinical isolate sequence and its infectious clone
An infectious cloning and sequence technology, applied in the fields of biology and genetic engineering, can solve the problem of no HuNoV infectious cloning, etc., and achieve the effect of simple construction method and high reliability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
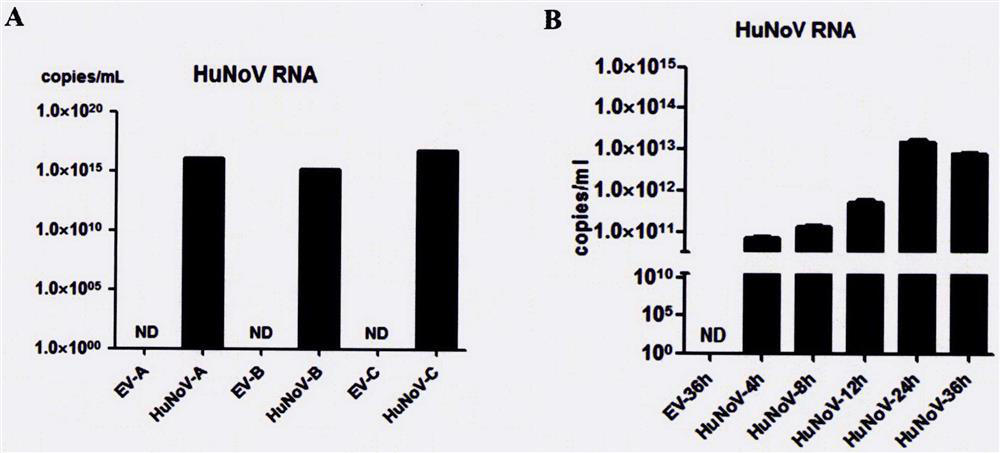
[0054] Example 1: Replication and toxin-producing ability of HuNoV infectious clones
[0055] Spread eukaryotic cells A, B, and C on 10 cm cell culture dishes, transfect 10 μg of pCMV-HuNoV plasmid into eukaryotic cells A, B, and C after 16 hours, and collect the cells after 3 days; or spread eukaryotic cells C on a 6-well plate 16 hours later, 3 μg of pCMV-HuNoV plasmid was transfected into eukaryotic cell C, and the cells were collected after 4, 8, 12, 24, and 36 hours of transfection; after centrifugation at 1100pm for 5 minutes:
[0056] Cell pellet:
[0057] 1. Take 107 cells and use the RNA extraction kit from MN Company to extract the total RNA in the cells.
[0058] 2. Using the RNA as a template, use the HuNoV GII nucleic acid detection kit of Sun Yat-Sen University Daan Gene Co., Ltd. to detect the replication of HuNoV RNA in various eukaryotic cells by real-time quantitative PCR (such as figure 2 );
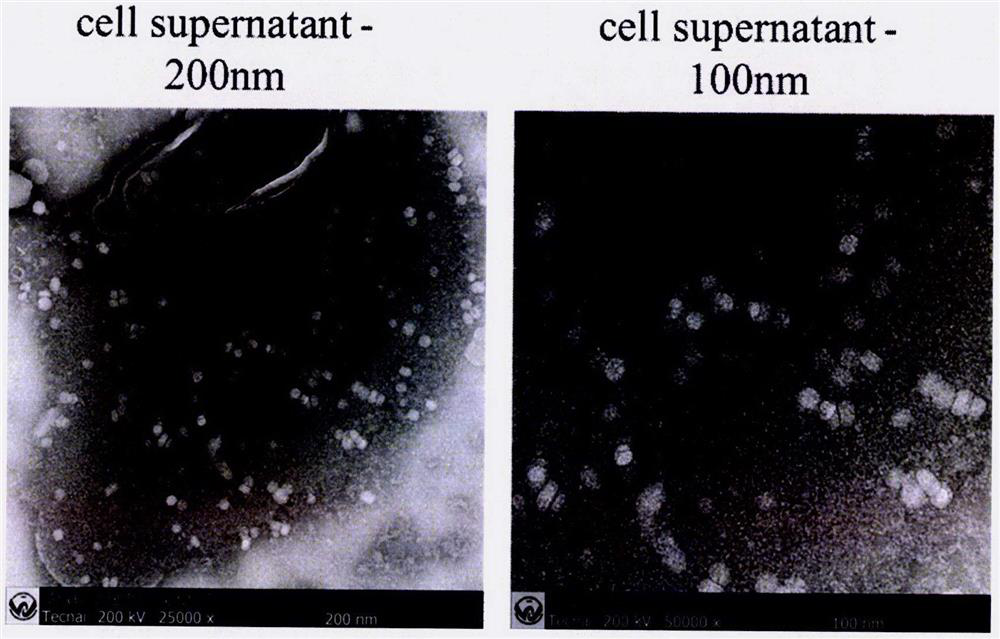
[0059] Cell supernatant:
[0060] 1. Use a 100KD ultrafiltra...
Embodiment 2
[0070] Example 2: Infectivity of Progeny Virions Produced by HuNoV Infectious Clones
[0071] 1. After transfecting C cells with 10 μg pCMV-HuNoV plasmid for 3 days, collect the cell supernatant, centrifuge at 1100 rpm for 5 minutes, pipette the cell supernatant into clean collection tubes, aliquot 1 mL in each tube and store in a -80°C refrigerator for later use.
[0072] 2. Spread 6-well plates with C cells, add 1 mL of virus supernatant to each well after 16 hours and infect for 4 hours, wash with 2 mL of 1×PBS for 3 times, then replace with fresh medium and culture for 24 hours.
[0073] 3. Remove the cell supernatant, add 2mL 1×PBS to each well to wash 3 times, scrape off the cells with a cell scraper, transfer to a 1.5mLEP tube, centrifuge at 1100rpm for 5min, remove the supernatant, and keep the cell pellet.
[0074] 4. Use the RNA extraction kit of MN company to extract the total RNA in the cells.
[0075] 5. Using the RNA as a template, use the HuNoV GII nucleic acid...
Embodiment 3
[0076] Example 3: Infectious replication ability of progeny virions produced by HuNoV infectious clones
[0077] 1. After transfecting C cells with 10 μg pCMV-HuNoV plasmid for 3 days, collect the cell supernatant, centrifuge at 1100 rpm for 5 minutes, pipette the cell supernatant into clean collection tubes, aliquot 1 mL in each tube and store in a -80°C refrigerator for later use.
[0078] 2. Spread 35mm glass-bottomed dishes with C cells, add 1mL virus supernatant to each well after 16 hours and infect for 4 hours, wash with 2mL 1×PBS for 3 times, then replace with fresh medium and culture for 24 hours.
[0079] 3. Remove the cell supernatant, add 2mL 1×PBS to each small dish and wash 3 times.
[0080] 4. Add 1 mL of 4% paraformaldehyde to each well and fix at room temperature for 10 min.
[0081] 5. Remove paraformaldehyde, add 1mL 1×PBS to each small dish and wash once.
[0082] 6. Remove PBS, add 1mL 2% TritonX-100 to each small dish, and permeabilize at room temperatu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com