A kind of nickel oxide and microcrystalline graphite composite and its preparation method and application
A technology of microcrystalline graphite and nickel oxide, which is applied in the direction of graphite, structural parts, electrical components, etc., can solve the problems of poor electrochemical performance, poor conductivity and stability, and fast capacity decay of materials, and achieve low raw material costs and improved The effect of conductivity and simple equipment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Step 1. Dissolve 1g of polyacrylonitrile in 9g of N,N-dimethylformamide (DMF), keep stirring at 75°C for 3 hours, and then stir at room temperature for 12 hours to form a viscous transparent solution. spinning solution. The prepared spinning solution was prepared by electrospinning equipment to prepare PAN nanofibers, and transferred to a high-temperature tube furnace for pre-oxidation reaction in an air atmosphere at 250 °C for 2 hours, and then at 700 °C under N 2 Atmosphere carbonization was carried out for 3 hours to obtain a carbon nanofiber film.
[0040] Step 2. Dissolve nickel acetate and NaOH in deionized water sequentially under stirring, and stir for 30 minutes to obtain a nickel salt solution, wherein the final concentration of nickel salt is 0.3 mol / L, and the final concentration of NaOH is 0.5 mol / L. Weigh the carbon nanofiber film obtained in step 1 according to 2% of the theoretically generated mass of NiO, and add it into the nickel salt solution to ob...
Embodiment 2
[0043] Step 1. Prepare a carbon nanofiber membrane according to the method in Example 1 for future use.
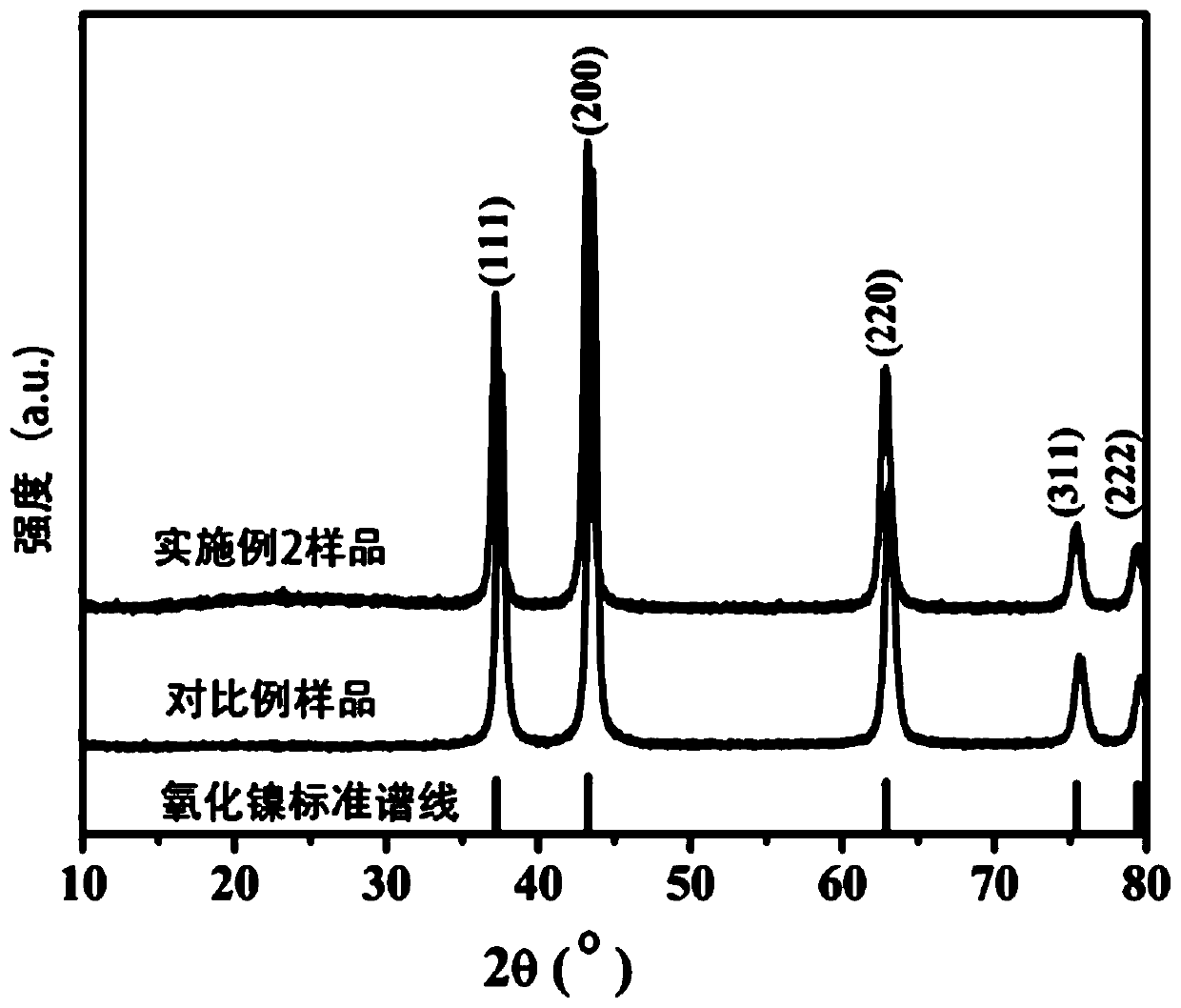
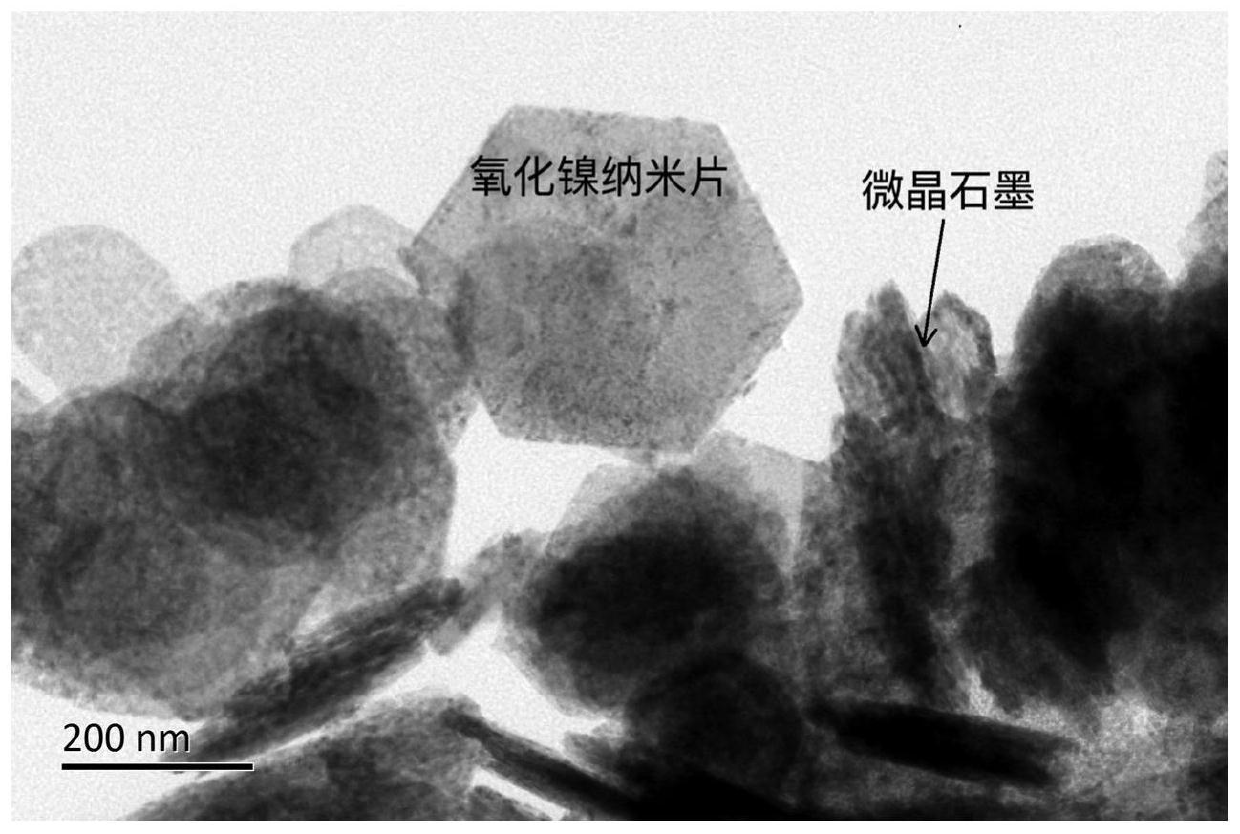
[0044] Step 2. Under stirring, 2.3g of nickel nitrate and 0.5g of NaOH were dissolved in 60mL of deionized water in turn, and stirred for 30min to obtain a nickel salt solution. The carbon nanofiber film was weighed according to 3% of the theoretically generated mass of NiO and added to the nickel salt solution to obtain a green suspension. The green suspension was transferred into a Teflon autoclave and hydrothermally treated at 200 °C for 8 h. After the reaction, the obtained sample was washed several times with deionized water and ethanol to completely remove nickel ions and sodium ions in the reactant. The precipitate was dried at 70 °C, and at 500 °C in N 2 Treated in the atmosphere for 2 hours (heating rate 4°C·min -1 ), after the reaction was completed, the furnace was cooled to obtain a composite of nickel oxide and microcrystalline graphite. The X-ray diffract...
Embodiment 3
[0047] Step 1. Prepare a carbon nanofiber membrane according to the method in Example 1 for future use.
[0048] Step 2. Dissolve nickel sulfate and NaOH in deionized water sequentially under stirring, and stir for 30 minutes to obtain a nickel salt solution, wherein the final concentration of nickel salt is 0.2 mol / L, and the final concentration of NaOH is 0.4 mol / L. Weigh the carbon nanofiber film obtained in step 1 according to 6% of the theoretically generated mass of NiO, and add it into the nickel salt solution to obtain a green suspension. The green suspension was transferred into a Teflon autoclave and hydrothermally treated at 200 °C for 12 h. After the reaction, the obtained sample was washed several times with deionized water and ethanol to completely remove nickel ions and sodium ions in the reactant. The precipitate was dried at 70 °C and N at 800 °C 2 Treated in the atmosphere for 2 hours (heating rate 4°C·min -1 ), after the reaction was completed, the furnac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com