Synthesizing method of 1,2,3-triazole compound of On-DNA in DNA coded compound storehouse
A technology for compound libraries and synthesis methods, which is applied in chemical instruments and methods, sugar derivatives, sugar derivatives, etc., can solve the problems of unstable azide reagents, few commercially available types, and limited applications, and achieves universal substrate availability. The effect of high adaptability, easy availability of reaction raw materials and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
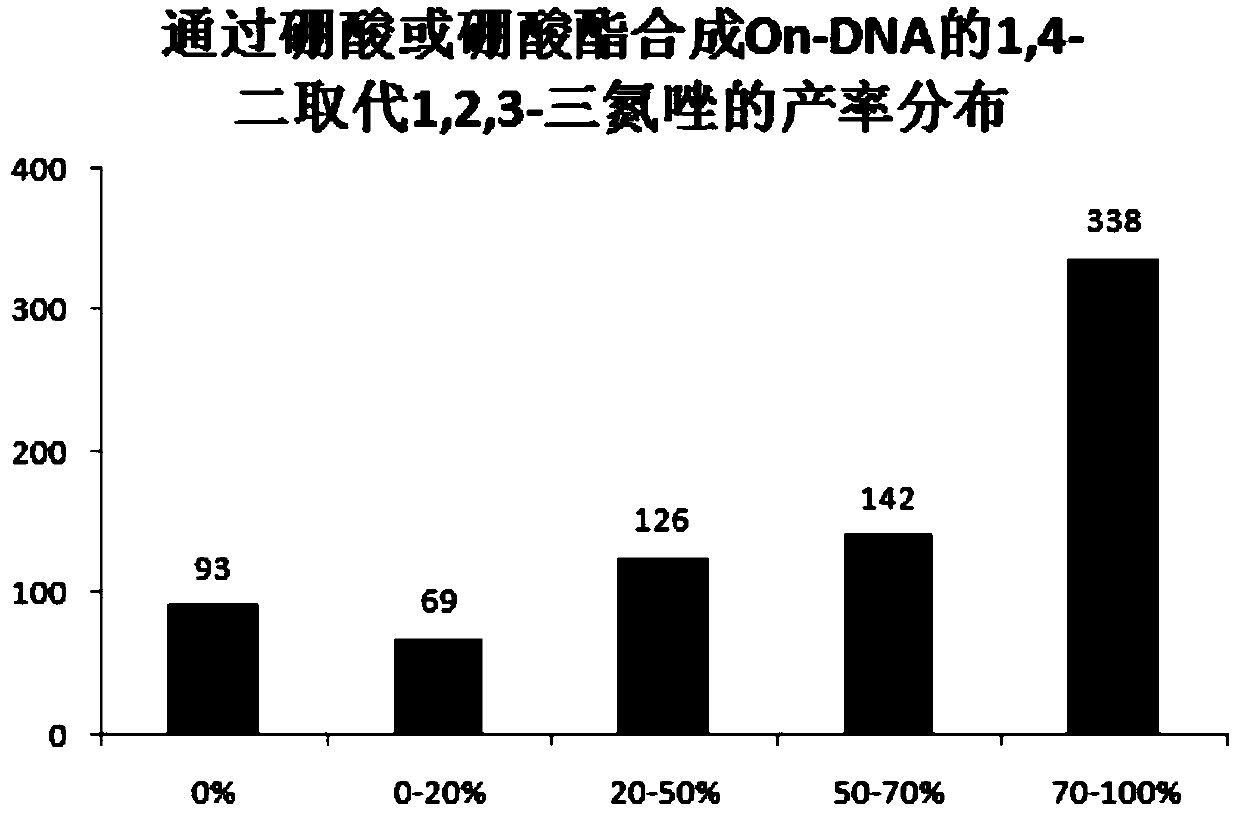
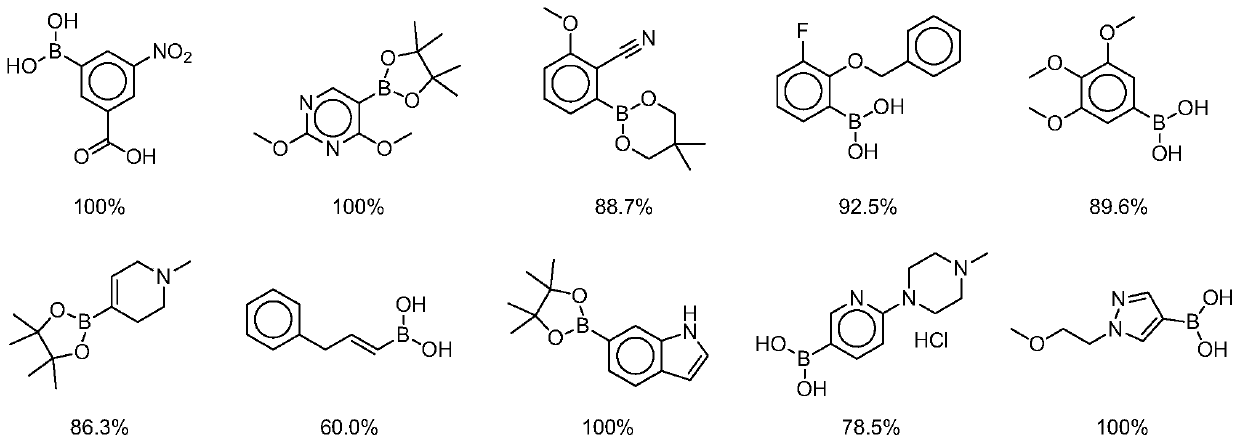
[0046] Example 1, On-DNA alkyne compound reacts with boronic acid or boric acid ester in the presence of copper catalyst and azide reagent to obtain On-DNA 1,4-disubstituted 1,2,3-triazole compound
[0047] 1) Synthesis of On-DNA alkyne compounds
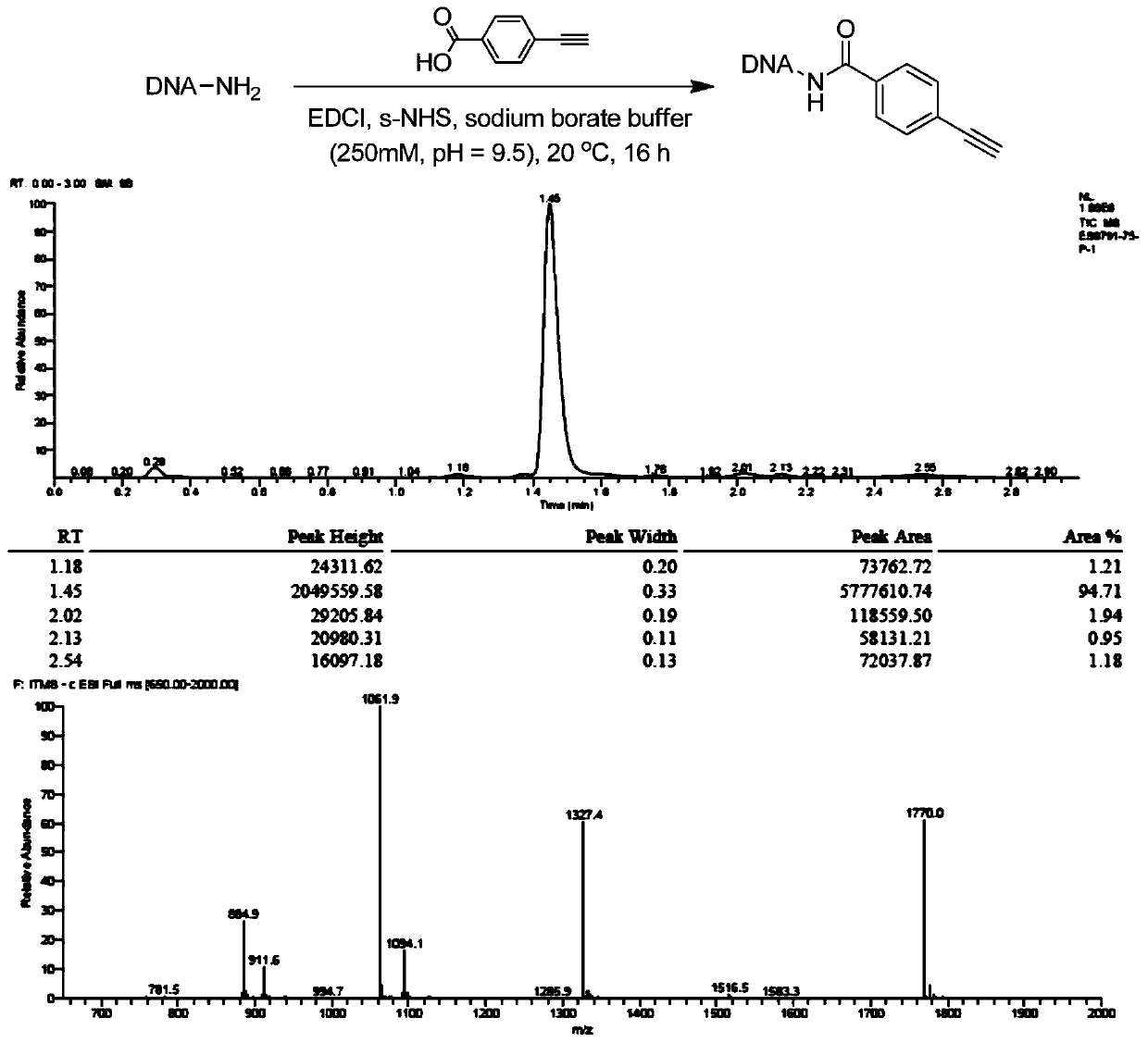
[0048] DNA-NH 2 (For example, the initial head fragment mentioned in patent CN108070009A) was dissolved in 250mM, pH=9.5 boric acid buffer solution, prepared into a 1mM concentration solution, and reacted with p-acetylenebenzoic acid using EDCI as a condensation agent and s-NHS condensation activator to obtain the corresponding On-DNA alkyne compounds (references: Nat.Chem., 2015,7,3,241, see figure 1 ), after the reaction is completed, ethanol precipitation treatment, concentration and drying are directly used for the next On-DNA cycloaddition reaction.
[0049]
[0050] The purity of the On-DNA alkyne compound after ethanol precipitation treatment was 95%.
[0051] 2) Synthesis of On-DNA 1,4-disubstituted 1,2,3-triazole comp...
Embodiment 2
[0059] Example 2, On-DNA azide compound is prepared in the presence of bis(cyclopentadiene) nickel and 4,5-bisdiphenylphosphine-9,9-dimethylxanthene 1, 5-disubstituted 1,2,3-triazole compounds
[0060] 1) Synthesis of On-DNA azide compounds
[0061] DNA-NH 2 (For example, the starting head fragment mentioned in patent CN108070009A) was dissolved in 250mM, pH=9.5 boric acid buffer solution, prepared into a 1mM concentration solution, and reacted with p-azidobenzoic acid using EDCI as a condensation agent and s-NHS condensation activator Obtain the corresponding On-DNA azide compound (references: Nat.Chem., 2015,7,3,241, see Figure 4 ), after the reaction is completed, ethanol precipitation treatment, concentration and drying are directly used for the next On-DNA cycloaddition reaction.
[0062]
[0063] The purity of the On-DNA azide compound after ethanol precipitation treatment was 93%.
[0064] 2) Synthesis of On-DNA 1,5-disubstituted 1,2,3-triazole compounds
[006...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com