Method for purifying penehyclidine
A technology of penehyclidine and phenylcyclopentyl oxirane, which is applied in the field of removing related substances in the synthesis of penehyclidine, can solve problems such as not being able to meet the purity requirements of penehyclidine, and achieve safety assurance and effectiveness, improved purity, and effective separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
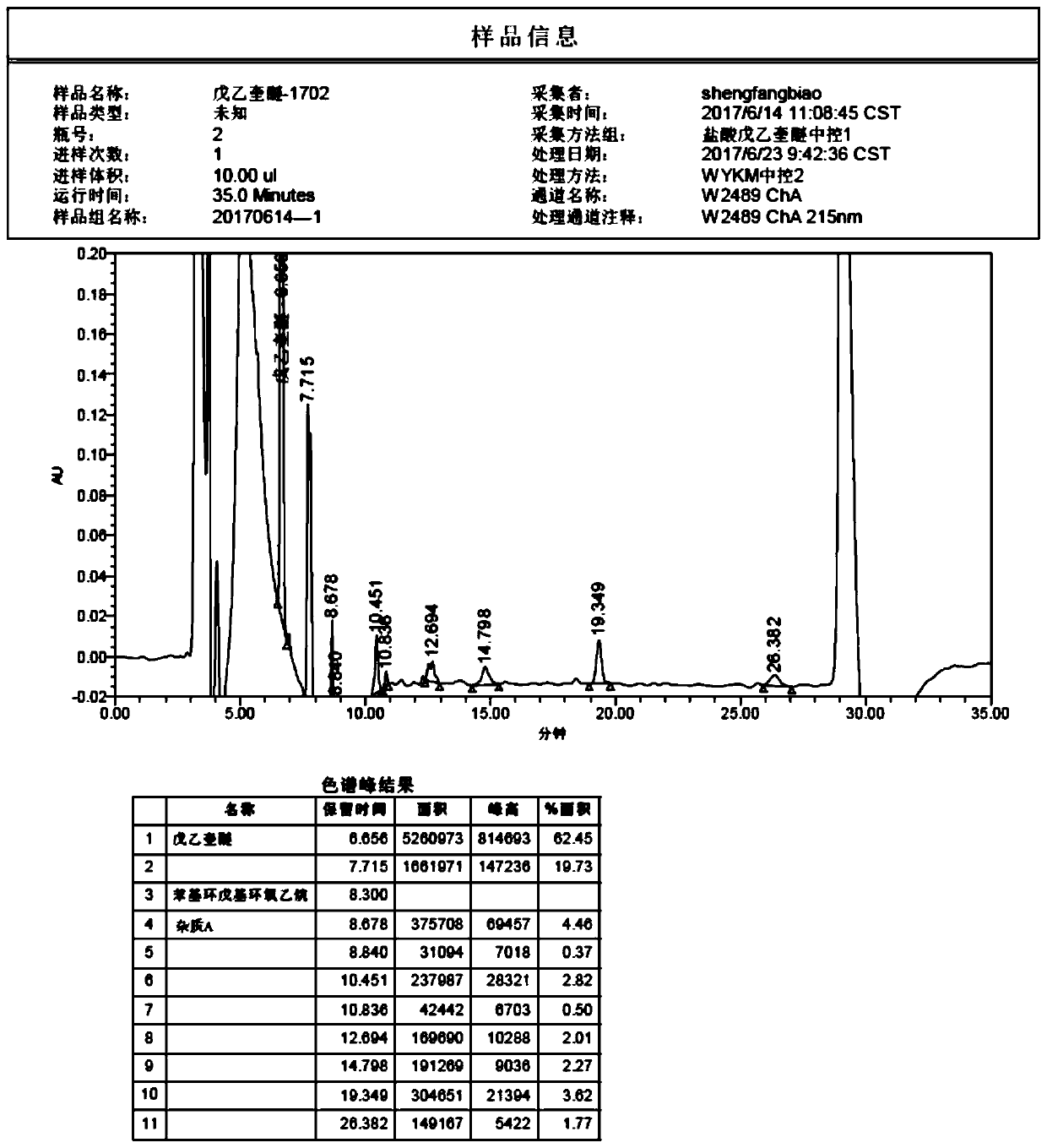
[0024] 300g phenylcyclopentyl oxirane, 3-quinine alcohol (the molar ratio of phenylcyclopentyl oxirane to 3-quinine alcohol is 1:1~1.2), 6L dimethyl sulfoxide and strong base reagent Synthesis of penhyclidine hydrochloride. After the reaction is completed in the central control detection, the reaction solution drops below 25°C, and the reaction solution is analyzed by HPLC, see figure 1 .
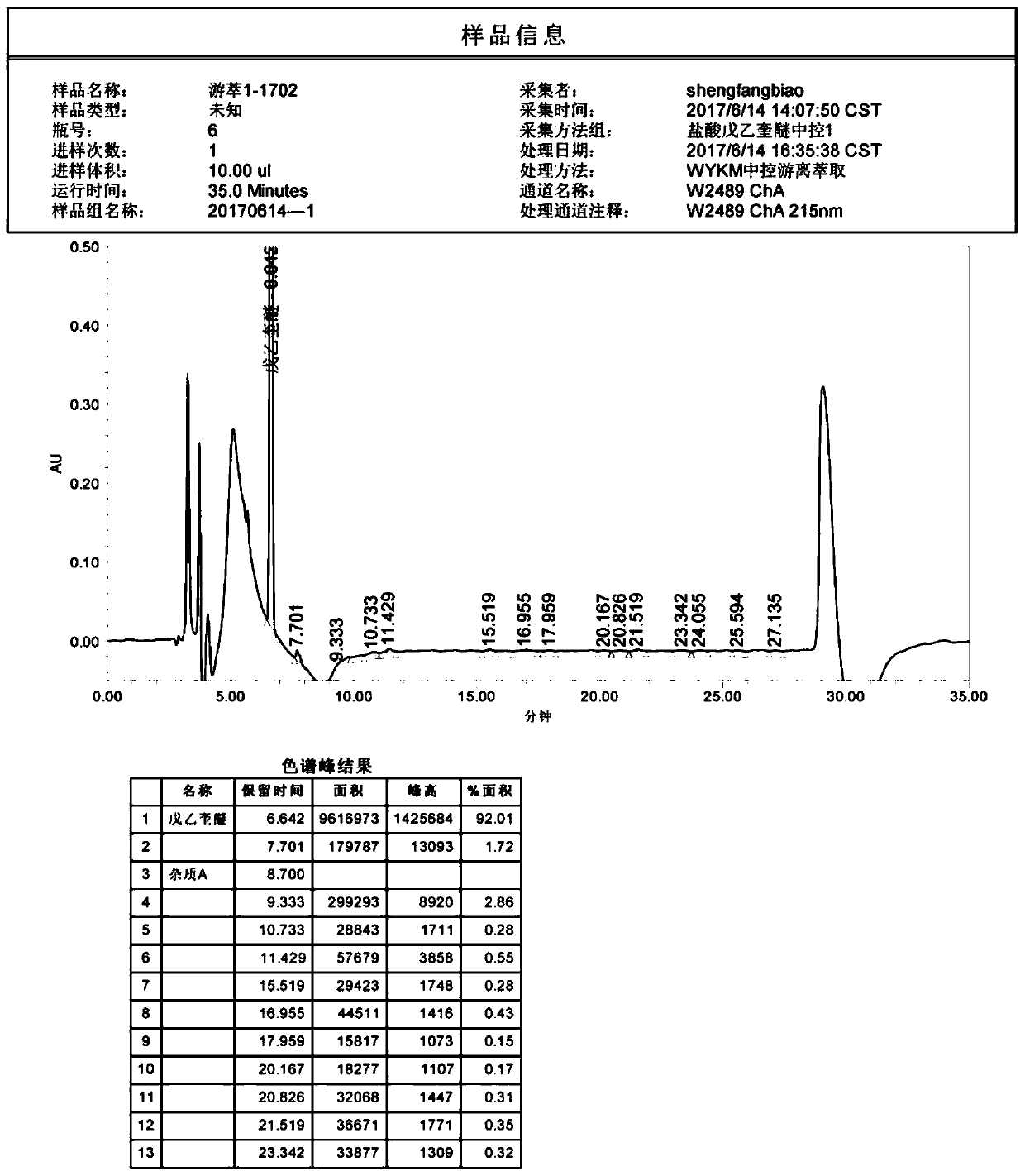
[0025] Add 6 kg of water to the reaction solution, add 12 L of methyl tert-butyl ether into the reaction kettle, stir for 15 minutes to 30 minutes, stop stirring, and let stand for more than 15 minutes. Separation, remove the upper organic phase, and the lower aqueous phase as waste liquid.
[0026] Add 9.75 L of 0.2 mol / L hydrochloric acid solution to the organic phase, stir for 15 minutes to 30 minutes, then stop stirring, and let stand for more than 15 minutes. Liquid separation. The lower aqueous phase was transferred to the reactor, and the upper organic phase was treated as waste ...
Embodiment 2
[0038] 150g phenylcyclopentyl oxirane, 3-quinine alcohol (the molar ratio of phenylcyclopentyl oxirane to 3-quinine alcohol is 1:1~1.2), 3L dimethyl sulfoxide and strong base reagent Synthesis of penhyclidine hydrochloride. After the central control detects that the reaction is complete, the reaction solution drops below 25°C.
[0039] Add 3 kg of water to the reaction solution, add 6 L of methyl tert-butyl ether into the reaction kettle, stir for 15 minutes to 30 minutes, stop stirring, and let stand for more than 15 minutes. Separation, remove the upper organic phase, and the lower aqueous phase as waste liquid.
[0040] Add 4.8 L of 0.2 mol / L hydrochloric acid solution to the organic phase, stir for 15 minutes to 30 minutes, stop stirring, and let stand for more than 15 minutes. Separation, the lower aqueous phase was transferred to the reactor, and the upper organic phase was treated as waste liquid.
[0041] Add 6L of methyl tert-butyl ether to the reaction kettle, adj...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com