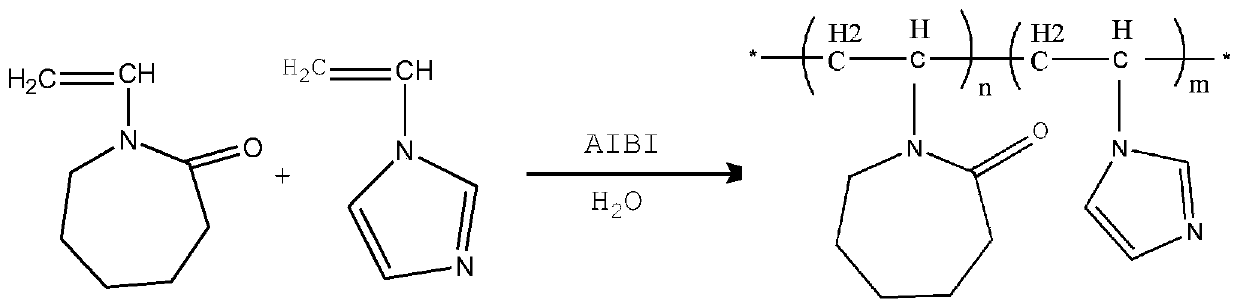
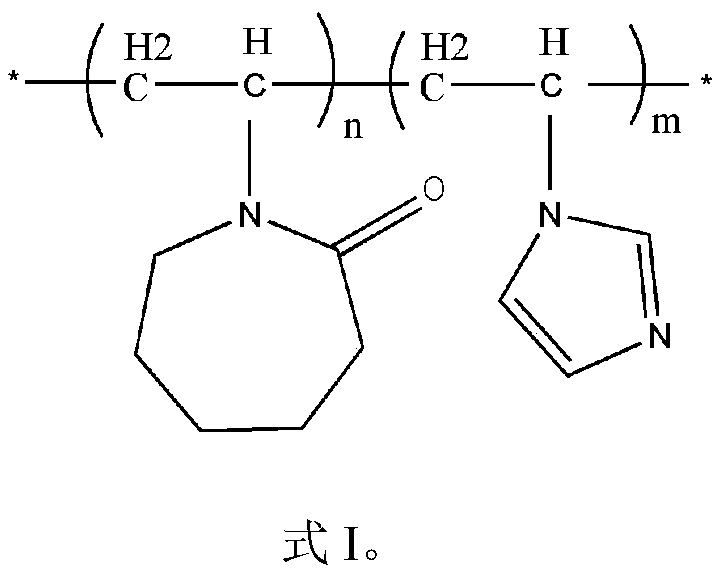
Composite hydrate kinetic inhibitor based on vinyl imidazole copolymer and application thereof
A kinetic inhibitor, vinylimidazole technology, applied in the field of composite hydrate kinetic inhibitor, can solve the problems of insufficient inhibitory effect, poor biodegradability of the copolymer, complicated production process, etc., to improve the overall inhibitory performance, water solubility, etc. and environmental protection improvement, the effect of a wide range of applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Such as figure 1 Shown, the preparation method of vinylimidazole copolymer comprises the steps:
[0036] Under nitrogen protection, 9.6 mL of vinylimidazole, 10 g of vinyl caprolactam, 0.068 g of azobisisobutylimidazoline hydrochloride and 100 mL of water were fully mixed in a 250 mL three-necked flask. The radical solution polymerization reaction was carried out at a stirring rate of 300 rpm and an oil bath of 80° C. for 7 hours, and the oil bath and stirring were turned off. After the solution was cooled to room temperature, the solution was transferred to a round bottom flask. The solution was rotary evaporated at 90°C until the precipitation was complete, cooled to room temperature, and then dropped into a large amount of cold anhydrous ether for precipitation, and the obtained solid was washed and dried in vacuum at 60°C for 24 hours.
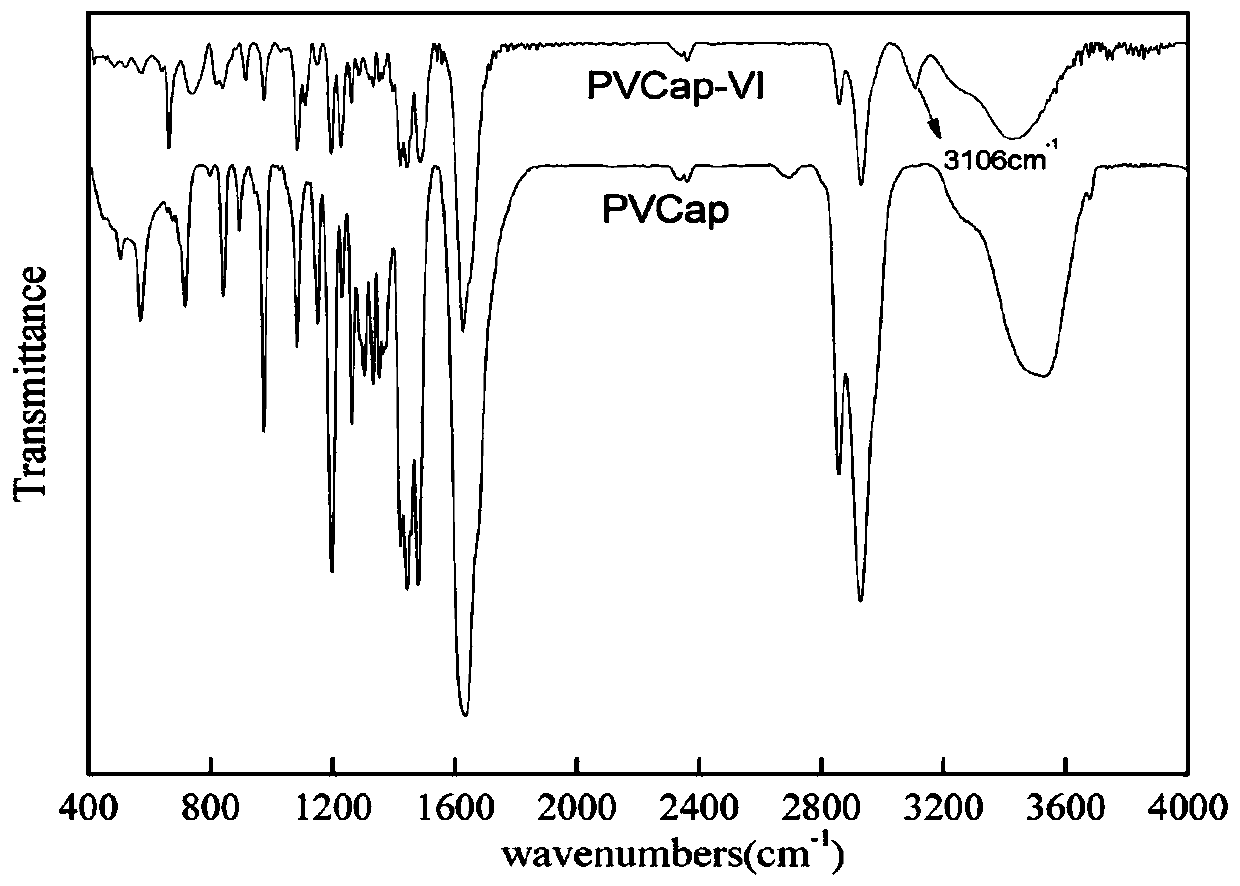
[0037] Fourier transform infrared spectroscopy and carbon nuclear magnetic resonance were used to characterize the characteristi...
Embodiment 2
[0039] The preparation method of vinylimidazole copolymer comprises the steps:
[0040] Under the protection of nitrogen, the monomer N-vinylcaprolactam, 1-vinylimidazole and deionized water were mixed uniformly in a three-necked flask, and the mass ratio of N-vinylcaprolactam to 1-vinylimidazole was 5:1. The adding amount of ionic water is 3:1 with the total mass ratio of polymer monomer, after fully stirring, dropwise add initiator azobisisobutylimidazoline hydrochloride (the quality of azobisisobutylimidazoline hydrochloride is 0.5% of the mass of deionized water), heated up to 80°C and reacted for 7 hours, then stopped the reaction to obtain the reaction liquid; when the reaction liquid was cooled to room temperature, it was dried by rotary evaporation at 90°C; after the precipitation was complete, drop into the precipitate Wash with a large amount of cold anhydrous ether, repeat the operation 3 times, place in a vacuum drying oven, and dry to constant weight to obtain pol...
Embodiment 3
[0043] The preparation method of vinylimidazole copolymer comprises the steps:
[0044] Under the protection of nitrogen, the monomer N-vinylcaprolactam, 1-vinylimidazole and deionized water were mixed uniformly in a three-necked flask, and the mass ratio of N-vinylcaprolactam and 1-vinylimidazole was 20:1. The addition of ionized water is 6:1 with polymer monomer mass ratio, after fully stirring, drip initiator azobisisobutylimidazoline hydrochloride (the quality of azobisisobutylimidazoline hydrochloride is de- 0.1% of the mass of ionic water), heated up to 95°C and reacted for 7 hours, then stopped the reaction to obtain the reaction solution; when the reaction solution was cooled to room temperature, it was dried by rotary evaporation at 90°C; after the precipitation was complete, a large amount of After washing with cold anhydrous ether and repeating the operation 3 times, place in a vacuum drying oven and dry to constant weight to obtain poly(vinyl caprolactam-vinylimida...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com