Double-target inhibitor as well as preparation method and application thereof
A dual-target and inhibitor technology, which can be used in pharmaceutical formulations, medical preparations containing active ingredients, organic active ingredients, etc., can solve the problems of unsatisfactory therapeutic effect of histone deacetylase inhibitors, and achieve good inhibition effect, the effect of enhancing inhibitory activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
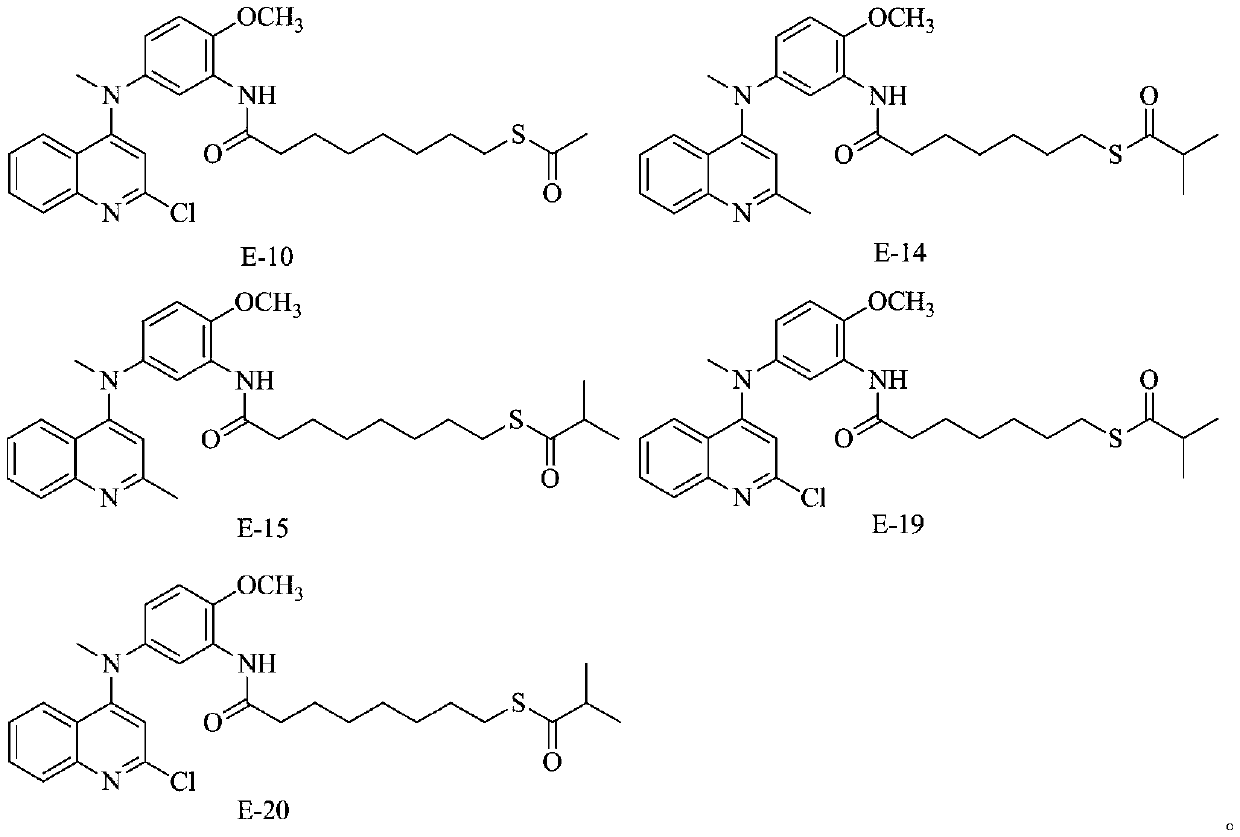
[0037]
[0038] 4-methoxy-N 1 -Methyl-N 1 -(2-Methyl-quinolyl-4-)-1,3-phenylenediamine (0.293 g, 1 mmol, 1 times the amount) was dissolved in 15 ml of anhydrous dichloromethane, and triethyl Amine (209 μl, 1.5 mmol, 1.5 times the amount) and 4-bromobutyryl chloride (0.278 g, 1.5 mmol, 1.5 times the amount), the mixture was stirred at 25 ° C for 4 hours, and the reaction mixture was directly used on silica gel Separation and purification by column chromatography, the eluent was dichloromethane:methanol=40:1, and 0.221 g of compound C-1 was obtained with a yield of 50%.
[0039] Compound C-1 (0.221 g, 0.5 mmol, 1 times the amount) and potassium thioacetate (0.114 g, 1 mmol, 2 times the amount) were mixed in 10 ml of absolute ethanol, and stirred at 25 ° C for 24 hours , directly separated and purified by silica gel column chromatography, the eluent was dichloromethane:methanol=40:1, and 98.5 mg of compound E-1 was obtained with a yield of 45%.
[0040] The NMR characteriza...
Embodiment 2
[0046]
[0047] The preparation method of Example 2 is basically the same as that of Example 1, except that 4-bromobutyryl chloride is replaced by 5-bromovaleryl chloride.
[0048] The NMR characterization data of compound E-2 are as follows:
[0049] 1 H-NMR(400MHz,DMSO-d6),δ9.02(brs,1H),8.29(m,1H),7.91(m,1H),7.71(m,1H),7.46(m,1H),7.35( s,1H),7.32(d,J=2.0Hz,1H),7.09(dd,J=8.8,2.0Hz,1H),7.02(d,J=8.8,1H),3.81(s,3H),3.53 (t,J=8.8Hz,2H),3.38(s,3H),2.64(s,3H),2.37(t,J=8.8Hz,2H),2.30(s,3H),1.82(m,2H) ,1.72(m,2H).
[0050] 13 C-NMR (101MHz, DMSO-d6), δ194.8, 179.8, 159.1, 149.2, 148.8, 141.6, 138.0, 129.6, 128.6, 127.2, 124.6, 123.7, 119.6, 115.3, 110.1, 106.5, 106.2, 51.33.0, 4 , 35.2, 28.5, 27.2, 24.7, 20.2.
[0051] The mass spectral data of compound E-2 are as follows:
[0052] HRMS: calcd for C 25 h 30 N 3 o 3 S + [M+H] + :452.2002found: 452.2004.
Embodiment 3
[0054]
[0055] The preparation method of Example 3 is basically the same as that of Example 1, except that 4-bromobutyryl chloride is replaced by 6-bromohexanoyl chloride.
[0056] The nuclear magnetic characterization data of compound E-3 are as follows:
[0057] 1 H-NMR(400MHz,DMSO-d6),δ9.02(brs,1H),8.29(m,1H),7.91(m,1H),7.71(m,1H),7.46(m,1H),7.35( s,1H),7.32(d,J=2.0Hz,1H),7.09(dd,J=8.8,2.0Hz,1H),7.02(d,J=8.8,1H),3.95(s,3H),3.50 (t,J=8.6Hz,2H),3.32(s,3H),2.62(s,3H),2.35(t,J=8.6Hz,2H),2.30(s,3H),1.86(m,2H) ,1.69(m,2H),1.29(m,2H).
[0058] 13 C-NMR (101MHz, DMSO-d6), δ194.8, 179.8, 159.1, 149.2, 148.8, 141.6, 138.0, 129.6, 128.6, 127.2, 124.6, 123.7, 119.6, 115.3, 110.1, 106.5, 106.2, 51.3, 38.0, 4 , 30.5, 29.5, 29.2, 25.3, 24.7, 20.0.
[0059] The mass spectral data of compound E-3 are as follows:
[0060] HRMS: calcd for C 26 h 32 N 3 o 3 S + [M+H] + :466.2159found: 466.2160.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com