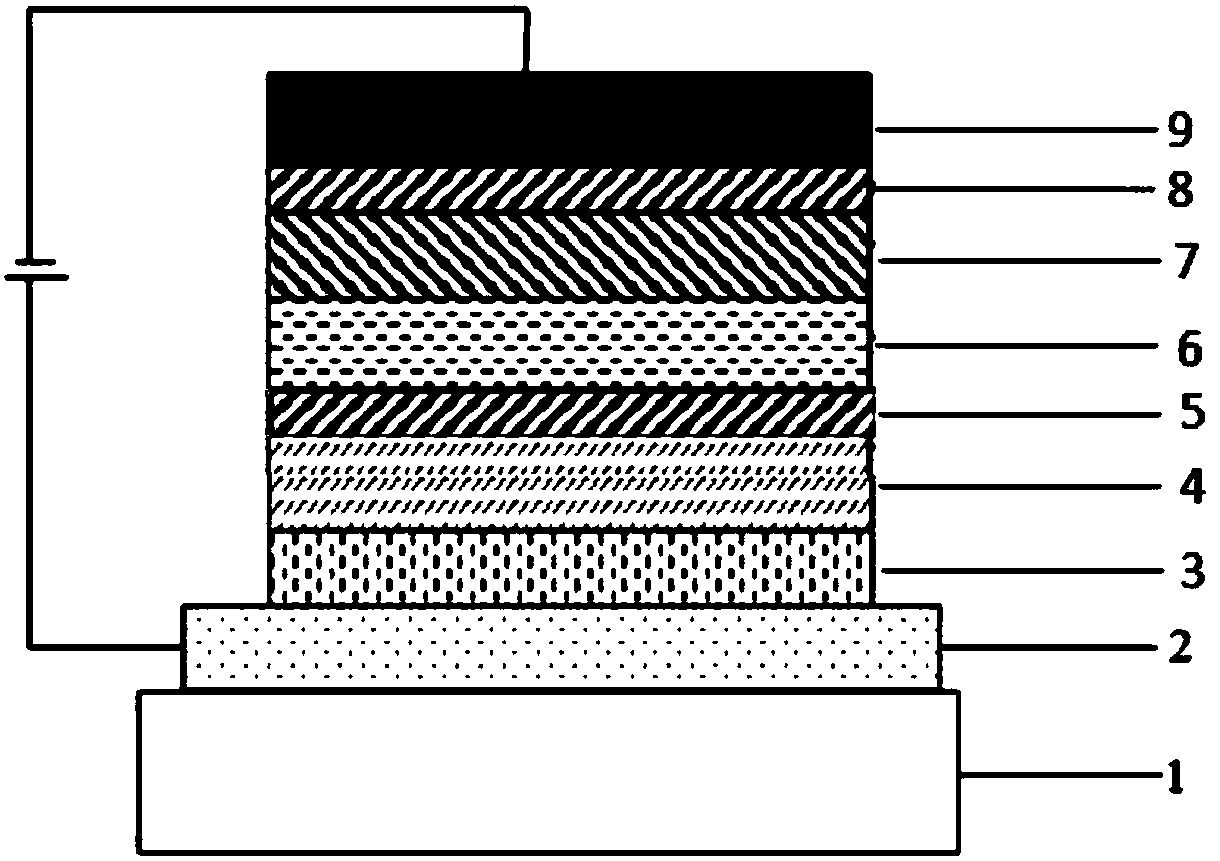
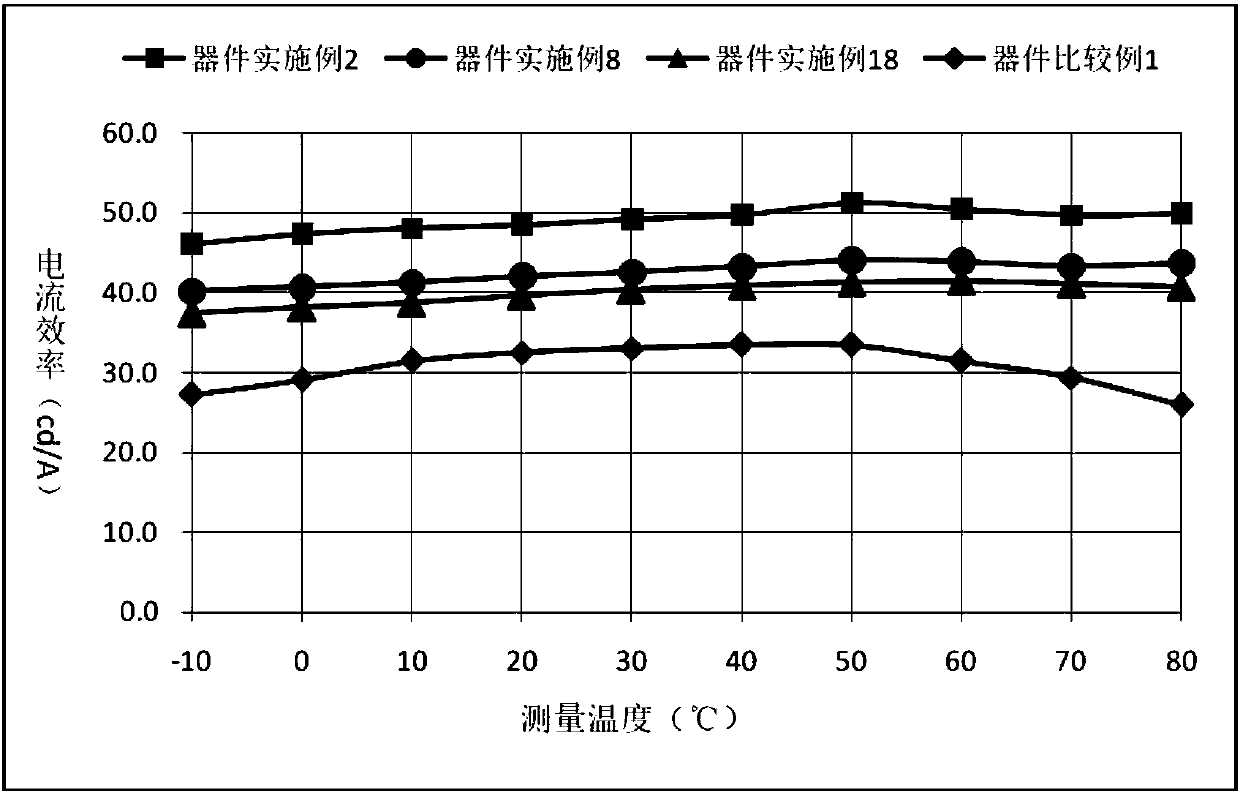
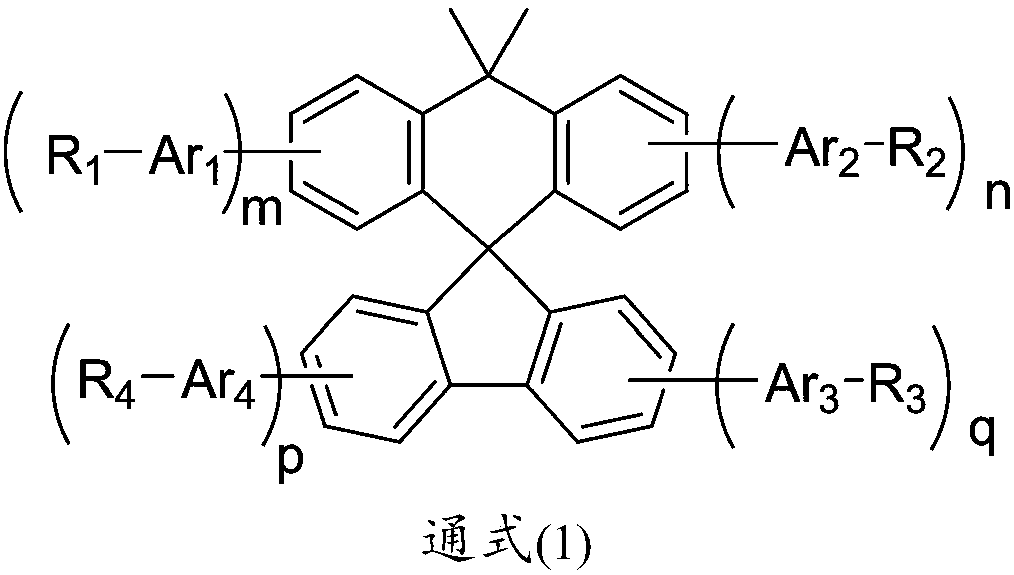
Compound with spirodimethyl anthracene fluorene as core and application thereof in organic light emission diode device
A technology of spirodimethyl anthracene fluorene and compound, which can be applied to the application field of organic electroluminescence devices, and can solve problems such as disparity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0138] Example 1 Compound 4
[0139]
[0140] In a 250ml three-necked flask, under the protection of nitrogen, add 0.01mol of intermediate A1, 0.012mol of raw material B1, and 150ml of toluene, stir and mix, then add 0.03mol of sodium tert-butoxide, 5×10 -5 molPd 2 (dba) 3 , 5×10 -5 mol tri-tert-butylphosphine, heated to 105 ° C, refluxed for 24 hours, sampling point plate, showed that no bromine remained, the reaction was complete; naturally cooled to room temperature, filtered, and the filtrate was subjected to vacuum rotary evaporation (-0.09 MPa, 85 ° C ), passed through a neutral silica gel column to obtain the target product, the HPLC purity was 99.3%, and the yield was 64.7%;
[0141] Elemental analysis structure (molecular formula C 43 H 35 N): Theoretical C, 91.29; H, 6.24; N, 2.48; Tested: C, 91.27; H, 6.25; N, 2.50. ESI-MS (m / z) (M+): theoretical value 565.28, found value 565.31.
Embodiment 2
[0142] Example 2 Compound 15
[0143]
[0144] In a 250ml three-necked flask, under the protection of nitrogen, add 0.01mol intermediate A2, 0.012mol raw material B2, 150ml toluene, stir and mix, then add 0.03mol sodium tert-butoxide, 5×10 -5 molPd 2 (dba) 3 , 5 × 10-5mol tri-tert-butylphosphine, heated to 105 ° C, refluxed for 24 hours, sampling point plate, showed that no bromine remained, the reaction was complete; naturally cooled to room temperature, filtered, and the filtrate was subjected to reduced pressure rotary evaporation (- 0.09MPa, 85°C), passed through a neutral silica gel column to obtain the target product, the HPLC purity was 99.3%, and the yield was 64.7%;
[0145] Elemental analysis structure (molecular formula C 40 H 29 NS): Theoretical C, 86.45; H, 5.26; N, 2.52; S, 5.77; Tested: C, 86.43; H, 5.21; N, 2.55; S, 5.75. ESI-MS (m / z) (M+): Theoretical value is 555.20, and the found value is 555.25.
Embodiment 3
[0146] Example 3 Compound 20
[0147]
[0148] In a 250ml three-necked flask, under the protection of nitrogen, add 0.01mol intermediate A3, 0.012mol raw material B3, 150ml toluene, stir and mix, then add 0.03mol sodium tert-butoxide, 5 × 10 -5 molPd 2 (dba) 3 , 5×10 -5 mol tri-tert-butylphosphine, heated to 105 ° C, refluxed for 24 hours, sampling point plate, showed that no bromine remained, the reaction was complete; naturally cooled to room temperature, filtered, and the filtrate was subjected to vacuum rotary evaporation (-0.09 MPa, 85 ° C ), passed through a neutral silica gel column to obtain the target product, the HPLC purity was 99.3%, and the yield was 64.7%;
[0149] Elemental analysis structure (molecular formula C 49 H 37 NO): Theoretical C, 89.74; H, 5.69; N, 2.14; O, 2.44; Tested: C, 89.72; H, 5.67; N, 2.15; O, 2.46. ESI-MS (m / z) (M+): theoretical value 655.29, found value 655.32.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com