Novel drug capsule processing method
A technology for capsule processing and medicine, applied in the directions of capsule delivery, pharmaceutical formulations, and non-active ingredients medical preparations, etc., can solve the problems of fragile or softened, unsatisfactory elasticity, short storage time, etc. The effect of extended period and reasonable moisture content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
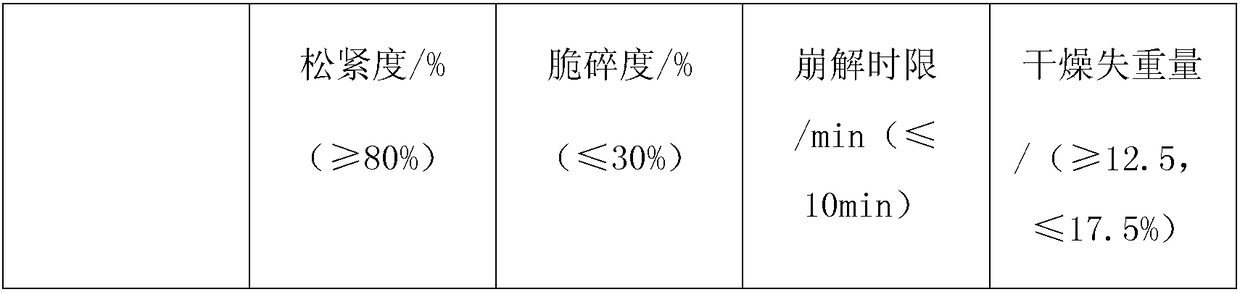
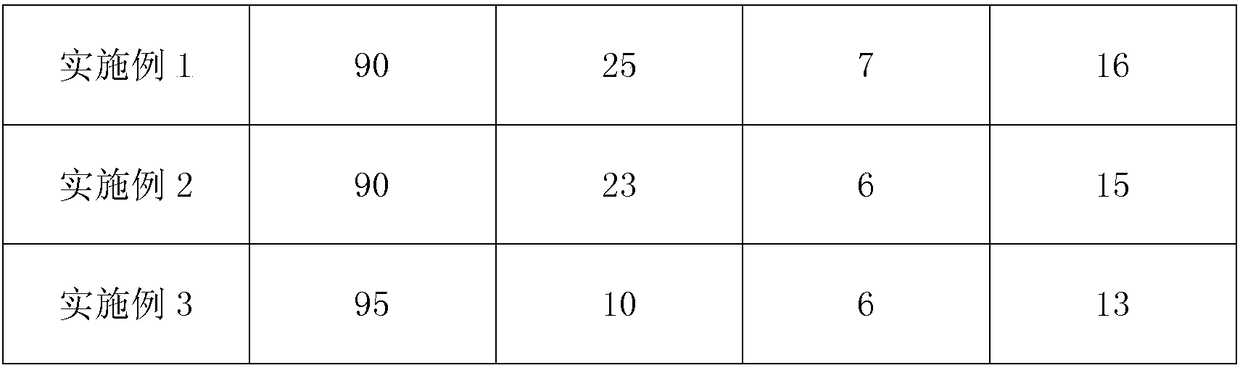
Examples
Embodiment 1
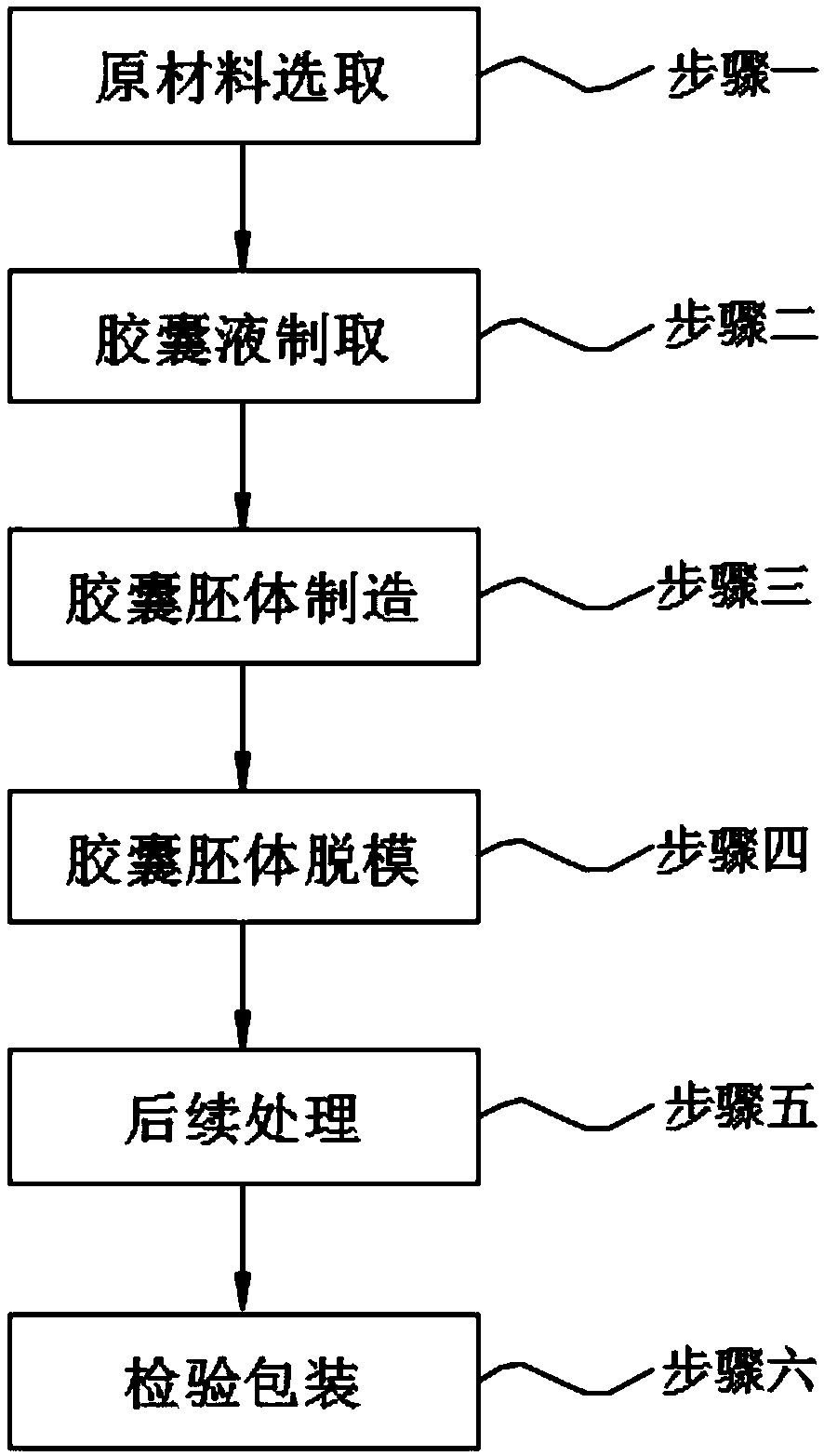
[0029] A novel pharmaceutical capsule processing method, comprising step 1, raw material selection; step 2, preparation of capsule liquid; step 3, manufacture of capsule body; step 4, demoulding of capsule body; step 5, subsequent processing; step 6, inspection of packaging;
[0030] In step one, according to the mass percentage of each component, it is respectively: 20% medicinal gelatin, 10% hypromellose, 15% starch mixture, 10% carrageenan, and 10% pectin , 5% gum tragacanth, 15% gum arabic and 15% alcohol mixture are selected, and weighed according to the sum of the percentages by weight being 1;
[0031] In step two, the preparation of the capsule liquid includes the following steps:
[0032] 1) Slowly add the starch mixture into a heatable container filled with pure water, and keep stirring, the stirring speed is 50-80rpm, the temperature is maintained at 50-60°C, and the weight of the pure water is 4% of the weight of the raw material except the alcohol mixture. -5 ti...
Embodiment 2
[0044] A novel pharmaceutical capsule processing method, comprising step 1, raw material selection; step 2, preparation of capsule liquid; step 3, manufacture of capsule body; step 4, demoulding of capsule body; step 5, subsequent processing; step 6, inspection of packaging;
[0045] In step one, according to the mass percentage of each component, it is respectively: 20% medicinal gelatin, 10% hypromellose, 15% starch mixture, 10% carrageenan, and 10% pectin , 7% gum tragacanth, 13% gum arabic and 15% alcohol mixture are selected, and weighed according to the sum of the percentages by weight being 1;
[0046] In step two, the preparation of the capsule liquid includes the following steps:
[0047] 1) Slowly add the starch mixture into a heatable container filled with pure water, and keep stirring, the stirring speed is 50-80rpm, the temperature is maintained at 50-60°C, and the weight of the pure water is 4% of the weight of the raw material except the alcohol mixture. -5 ti...
Embodiment 3
[0059] A novel pharmaceutical capsule processing method, comprising step 1, raw material selection; step 2, preparation of capsule liquid; step 3, manufacture of capsule body; step 4, demoulding of capsule body; step 5, subsequent processing; step 6, inspection of packaging;
[0060] In step one, according to the mass percentage of each component, it is respectively: 20% medicinal gelatin, 10% hypromellose, 15% starch mixture, 10% carrageenan, and 10% pectin , 8% gum tragacanth, 12% gum arabic and 15% alcohol mixture are selected, and weighed according to the sum of the percentages by weight being 1;
[0061] In step two, the preparation of the capsule liquid includes the following steps:
[0062] 1) Slowly add the starch mixture into a heatable container filled with pure water, and keep stirring, the stirring speed is 50-80rpm, the temperature is maintained at 50-60°C, and the weight of the pure water is 4% of the weight of the raw material except the alcohol mixture. -5 ti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com