Lidiastan related compounds and their preparation methods and uses
A compound and concentrate technology, applied in the field of rivaroxaban-related compounds and their preparation and application, can solve problems such as failure to effectively control and failure to establish a comprehensive and reliable quality control system for rivaroxaban
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043]
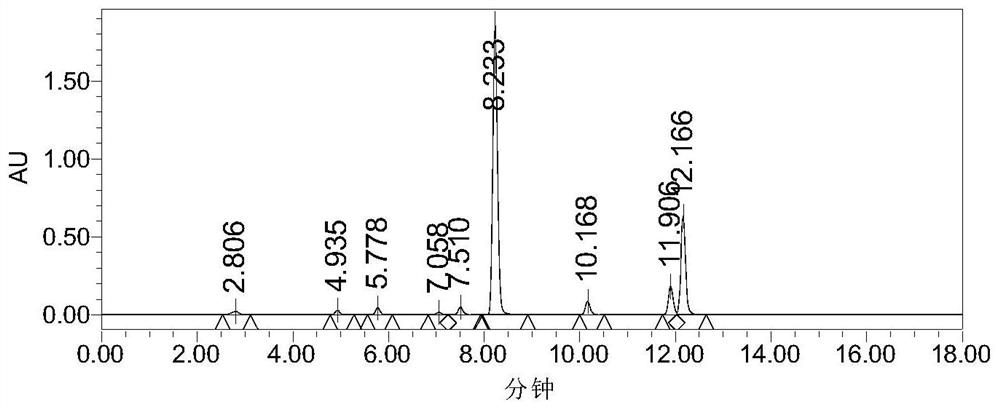
[0044] Add absolute ethanol (90 mL) and compound 1 (20 g) into the reaction flask, heat to 80° C., stir continuously for 30 h, and concentrate to dryness to obtain a concentrate. The concentrate was subjected to silica gel column chromatography (mobile phase dichloromethane:methanol=100:1), and 2.8 g of the compound of formula (II) was obtained by isolation (yield 11.4%).
[0045] The detection spectrum of the compound of formula (II) is as follows.
[0046] 1 H NMR (600MHz, DMSO-d 6 ):δ7.82-7.87(m,4H),5.08(d,1H),3.90-3.92(m,1H),3.55-3.62(m,2H),3.31-3.41(m,4H),1.01(t ,3H); MS(m / z):250.1[M+H] + .
Embodiment 2
[0048]
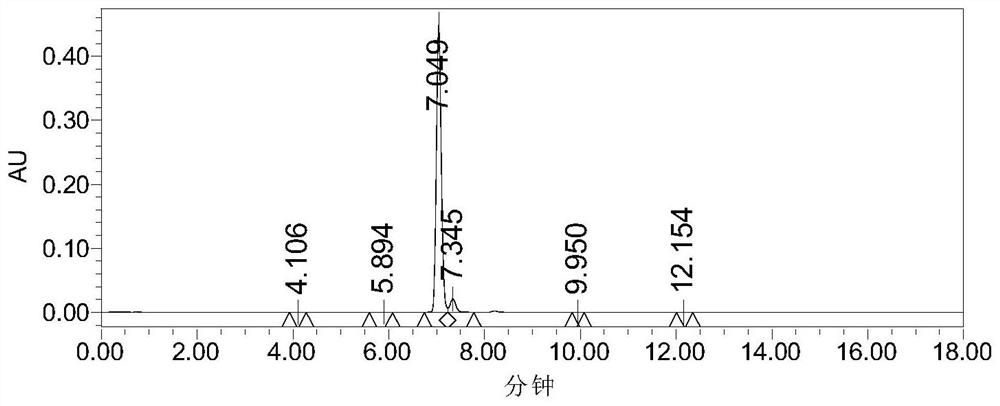
[0049] Put the compound of formula (II) (1g), 40% methylamine aqueous solution (0.85g) and absolute ethanol (8mL) into the reaction flask, react at 65°C for 3h and then cool down to room temperature, add 16% dilute hydrochloric acid to the reaction solution (2mL), crystallized at 0°C for 3h, then suction filtered, and concentrated the mother liquor to dryness to obtain a concentrate. Add purified water (5 mL), ethyl acetate (5 mL) and triethylamine (0.7 g) to the concentrate, add 5-chlorothiophene-2-formyl chloride (0.6 g) dropwise at room temperature, and stir at room temperature for 1 h after the addition , and then raised to 60 ° C for 1 h. The phases were separated, the organic phase was collected, and concentrated to dryness to obtain a concentrate. The concentrate was separated by silica gel column chromatography (mobile phase dichloromethane:methanol=100:1), and 0.3 g of the compound of formula (I) was obtained (purity 94%, yield 28.4%).
[0050] The detec...
Embodiment 3
[0054]
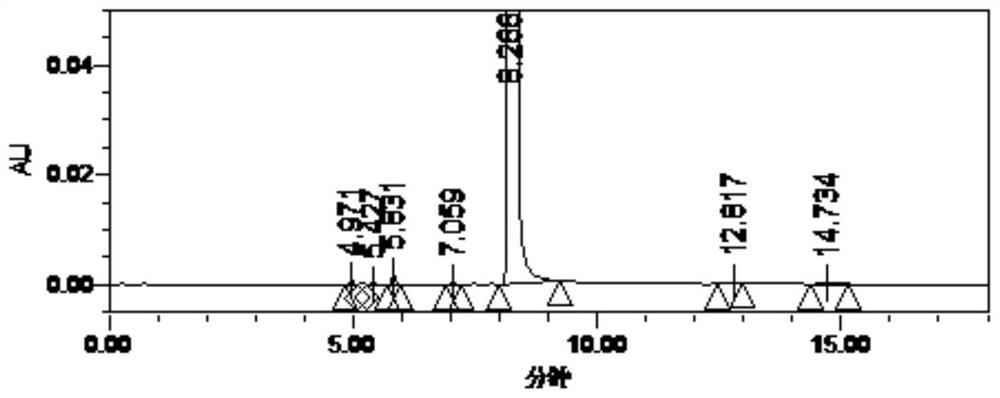
[0055] The compound of formula (IV) (0.8 g) was added to ammonia solution in methanol (7 M, 23 mL), stirred at room temperature for 15 h, and concentrated to dryness under reduced pressure to obtain a concentrate. Add purified water (11 mL), ethyl acetate (11 mL) and triethylamine (1.5 g) to the concentrate, add 5-chlorothiophene-2-formyl chloride (1.3 g) dropwise at room temperature, and stir at room temperature for 1 h after the addition , and then raised to 60 ° C for 1 h. The phases were separated, the organic phase was collected, and concentrated to dryness to obtain a concentrate. The concentrate was subjected to silica gel column chromatography (mobile phase dichloromethane:methanol=100:1), and 1.7 g of the compound of formula (I) was obtained (yield 81.0%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com