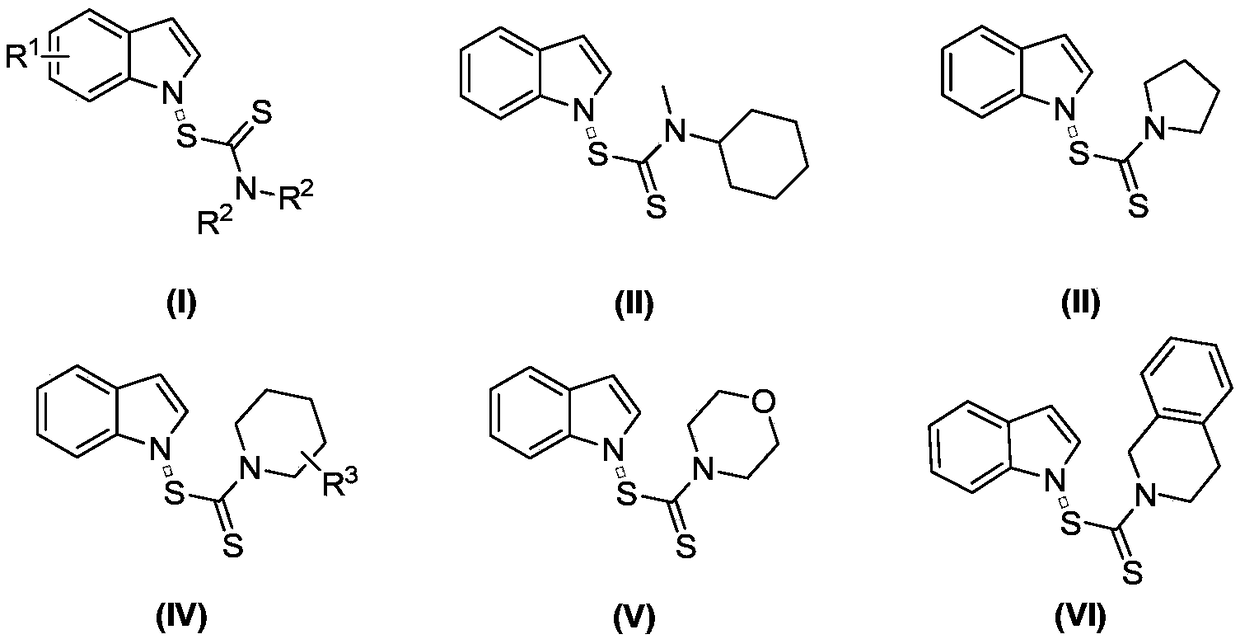
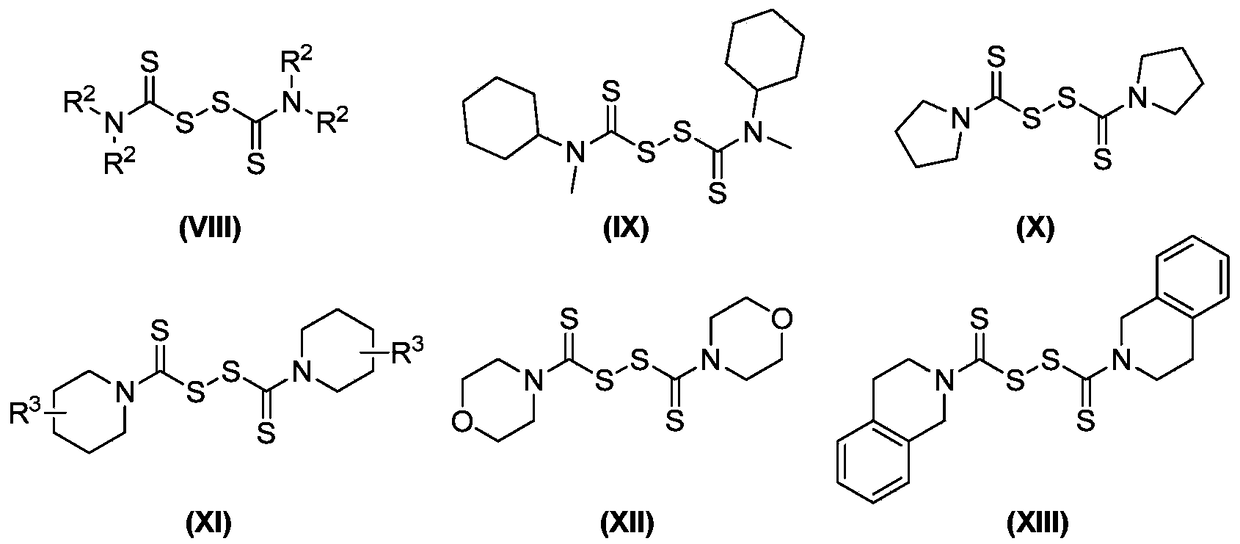
Preparation method of N-dithiocarbamate indole compound
A technology of dithiocarbamic acid and compounds, applied in the direction of organic chemistry, to achieve the effects of high yield, mild reaction conditions and short reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Indole (0.2mmol), N,N,N',N'-tetramethylthiuram (0.22mmol), potassium tert-butoxide (0.4mmol) and DCE (2.0mL) were added to a 5mL reaction flask respectively, Stir at room temperature. TLC tracking detection reaction. After 1 hour, the reaction was stopped. Water and ethyl acetate were added to the reaction system, the organic layer was separated, and the aqueous layer was washed three times with ethyl acetate. Combine all organic layers, dry with anhydrous sodium sulfate, concentrate, and separate by column chromatography (17% ethyl acetate petroleum ether solution), obtain product 43.9mg, yield rate is 93%, reaction process is shown in the following formula:
[0027]
[0028] Carry out nuclear magnetic resonance analysis to the product that present embodiment prepares:
[0029] 1 H NMR (500MHz, CDCl 3 )δ7.65(d, Jδ7.69(d, J=7.7Hz), 7.40(dt, J=17.7, 9.2Hz), 7.27(dd, J=13.1, 5.6Hz), 7.17(d, J=3.1 Hz), 6.79(d, J=3.0Hz), 5.51–4.77(m).=7.7Hz, 1H), 7.45(d, J=8.1Hz, 1...
Embodiment 2
[0037] Add indole (0.2mmol), N,N,N',N'-tetramethylthiuram (0.22mmol), potassium tert-butoxide (0.4mmol) and toluene (2.0mL) respectively into a 5mL reaction flask, Stir at room temperature. TLC tracking detection reaction. After 1 hour, the reaction was stopped. Water and ethyl acetate were added to the reaction system, the organic layer was separated, and the aqueous layer was washed three times with ethyl acetate. Combine all organic layers, dry with anhydrous sodium sulfate, concentrate, and separate by column chromatography (17% ethyl acetate petroleum ether solution), obtain product 37.3mg, productive rate is 79%, and reaction process is shown in the following formula:
[0038]
[0039] Carry out nuclear magnetic resonance analysis to the product that present embodiment prepares:
[0040] 1H NMR (500MHz, CDCl 3 )δ7.65(d, Jδ7.69(d, J=7.7Hz), 7.40(dt, J=17.7, 9.2Hz), 7.27(dd, J=13.1, 5.6Hz), 7.17(d, J=3.1 Hz), 6.79(d, J=3.0Hz), 5.51–4.77(m).=7.7Hz, 1H), 7.45(d, J=8...
Embodiment 3
[0042] Indole (0.2mmol), N,N,N',N'-tetramethylthiuram (0.22mmol), potassium tert-butoxide (0.4mmol) and 1,4-dioxo Hexacyclic (2.0 mL), stirred at room temperature. TLC tracking detection reaction. After 2 hours, starting material remained, but no more product. Water and ethyl acetate were added to the reaction system, the organic layer was separated, and the aqueous layer was washed three times with ethyl acetate. Combine all organic layers, dry with anhydrous sodium sulfate, concentrate, and separate by column chromatography (17% ethyl acetate petroleum ether solution), obtain product 25.0mg, productive rate is 53%, and reaction process is shown in the following formula:
[0043]
[0044] Carry out nuclear magnetic resonance analysis to the product that present embodiment prepares:
[0045] 1 H NMR (500MHz, CDCl 3 )δ7.65(d, Jδ7.69(d, J=7.7Hz), 7.40(dt, J=17.7, 9.2Hz), 7.27(dd, J=13.1, 5.6Hz), 7.17(d, J=3.1 Hz), 6.79(d, J=3.0Hz), 5.51–4.77(m).=7.7Hz, 1H), 7.45(d, J=8....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com