Novel embedded type eight-component medicinal powder with whitening and freckle removing functions and preparation method thereof
A whitening and freckle-removing, encapsulating technology, applied in skin care preparations, medical preparations containing active ingredients, pharmaceutical formulas, etc., to achieve good whitening effects, stable properties, and overcoming irritation effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] (1) Preparation of Xinbabaisan Extract: Most of the tyrosinase inhibitors extracted from natural products contain polar groups such as carboxyl and hydroxyl groups. Ethanol with different volume fractions is selected as the extraction solvent, and the optimal composition ratio is selected. , fixed solid-liquid ratio and other extraction conditions, the evaluation indexes were tyrosinase inhibition rate and DPPH free radical scavenging rate. Weigh each 10g of silkworm, Tribulus terrestris, Bletilla striata, Poria cocos, Cortex mulberry, Poria radix, Paeoniae Alba and pearl powder in the optimal proportion, 8 parts in total, use 35% ethanol, 50% ethanol, 65% ethanol in sequence , 80% ethanol, 95% ethanol, and absolute ethanol are used as extraction solvents for extraction, and the amount of extraction solvent used each time is 100 parts by volume, and each extraction is performed for 4 hours; Concentrate under pressure to prepare 10mL of liquid medicine (namely Xinbabaisa...
Embodiment 2
[0046] Contrastive experiment of encapsulated Xinbabaisan extract and unencapsulated extract.
[0047] 1. Comparative experiment on inhibition of tyrosinase between encapsulated Xinbabaisan extract and unencapsulated extract: with a mass concentration of 8 μg / mL encapsulated Xinbabaisan extract and unencapsulated Xinbabaisan extract , with L-tyrosine solution (1.5mmol / L) as substrate, with the concentration of 1.42mmol / L vitamin C as reference substance, measure the effect of wrapped type Xinbabaisan extract and unwrapped Xinbabaisan extract on tyrosine Inhibition rate of amino acid monophenolase activity.
[0048] With the same mass concentration of Xinbabaisan extract and wrapped type Xinbabaisan extract, with L-dopa solution (1.5mmol / L) as substrate, with the concentration of vitamin C as 1.42mmol / L as reference substance, determine Inhibition rate of packaged Xinbabaisan extract and uncoated Xinbabaisan extract on tyrosinase diphenolase. The results are shown in Table 2:...
Embodiment 3
[0057] Stability Evaluation of Packaged Xinbabai Powder
[0058] 1. Evaluation of thermal stability
[0059] The wrapped Xinbabaisan extract prepared in Example 1 and the unwrapped extract were respectively placed in transparent glass bottles and placed in a constant temperature test box at 48°C, and the appearance of the product was observed every 3 days.
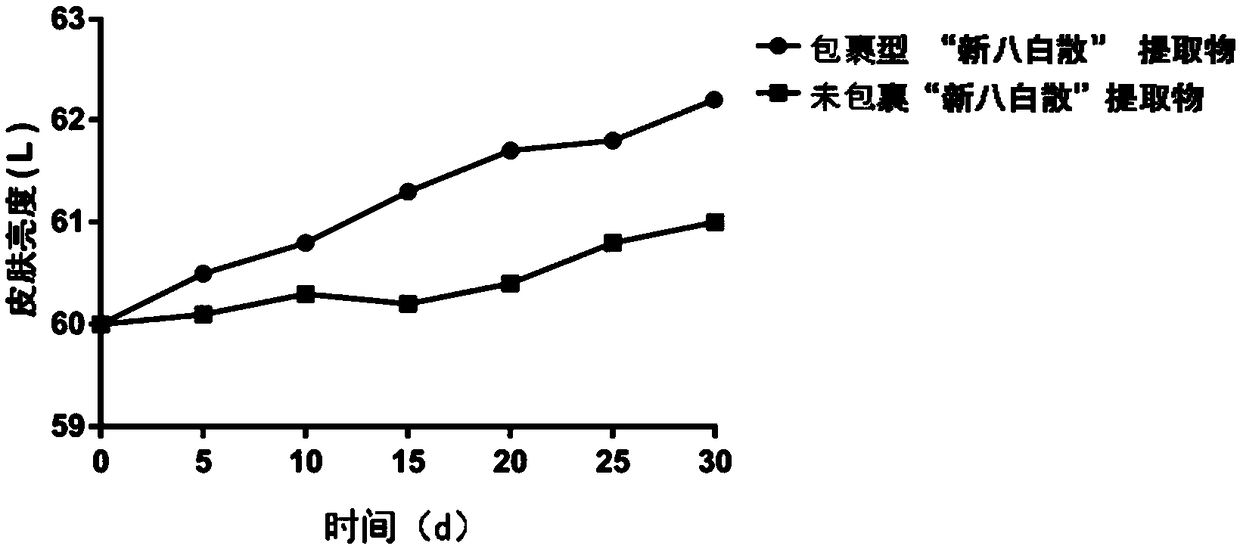
[0060] The experimental results show that the color of the unwrapped extract starts to change from white to slightly yellow in 8 days, and gradually deepens in color as time goes by; the wrapped type Xinbabaisan extract is continuously observed for 25 days, and the color of the product is uniform. Still remain white, there is no phenomenon of color change. This shows that after liposome encapsulation, the heat-resistant stability of the whitening and freckle-removing ingredients is greatly improved.
[0061] 2. Light stability evaluation
[0062] The wrapped Xinbabaisan extract prepared in Example 1 and the unwrapped ex...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com