AAV vectors for treatment of dominant retinitis pigmentosa
A vector and sequence technology, applied in the field of AAV vector for the treatment of dominant retinitis pigmentosa, can solve problems such as blindness and lack of approval
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
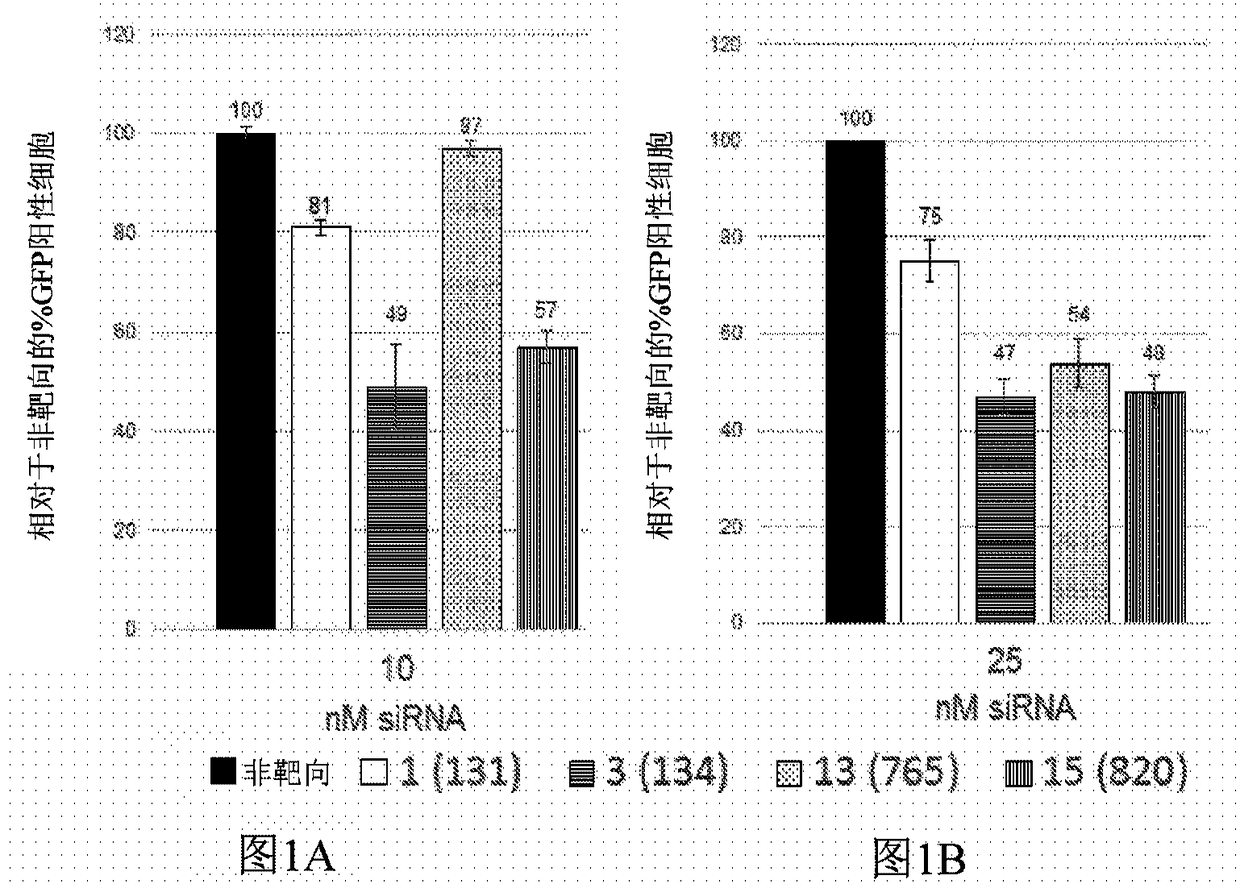
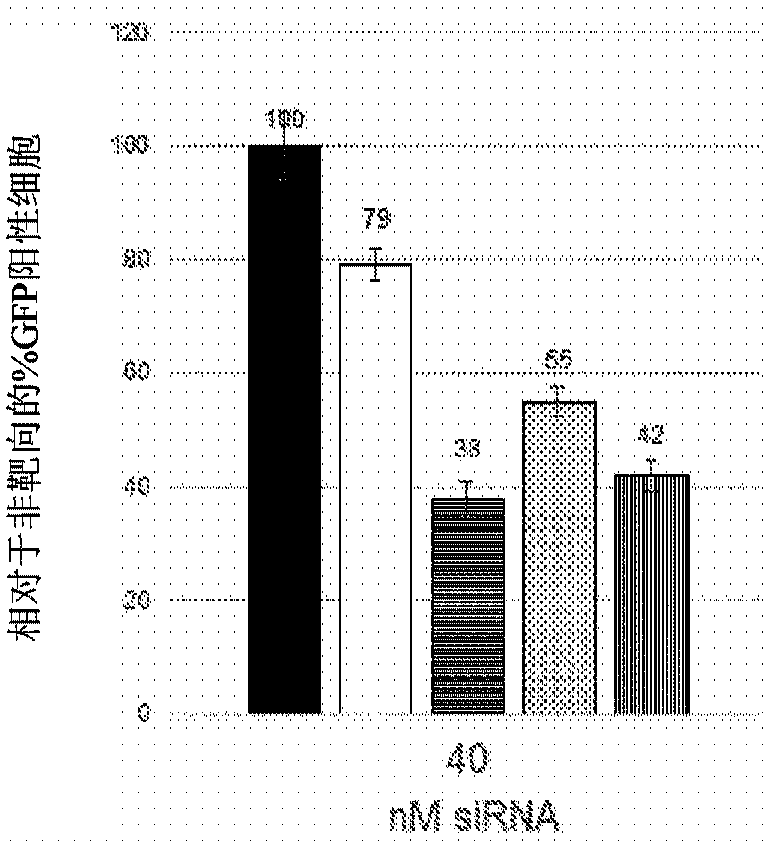
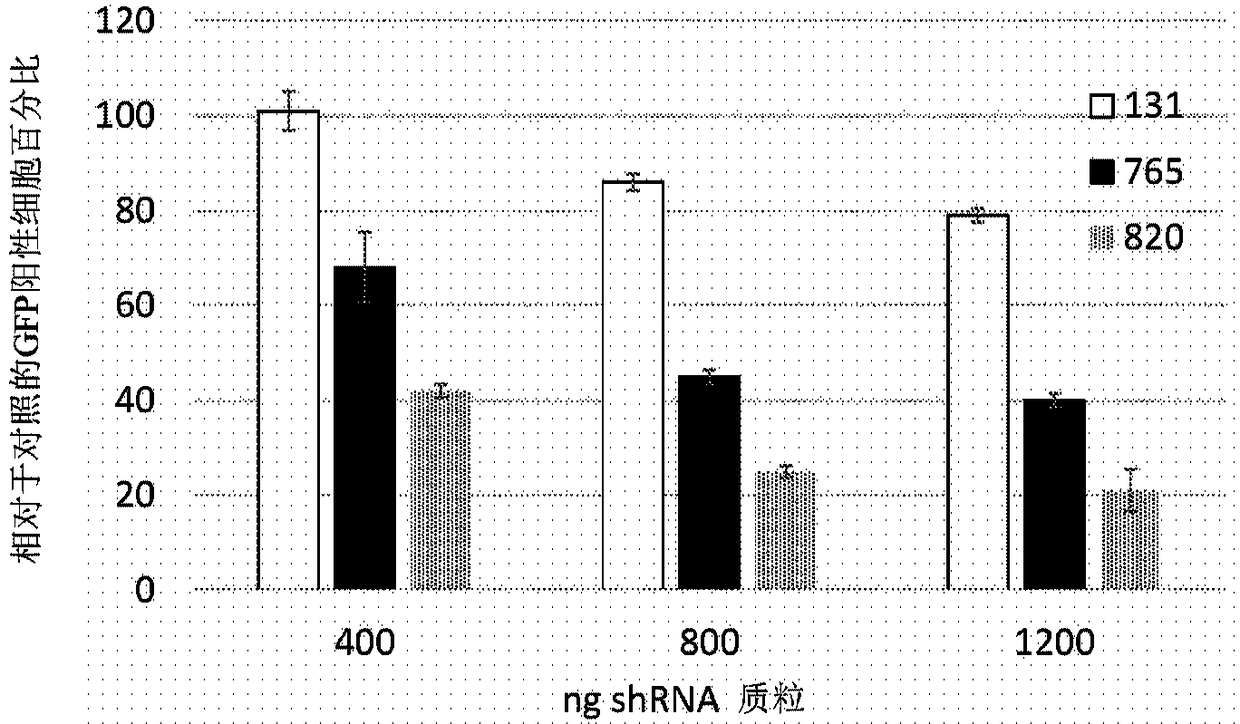
[0103] Example 1: Identification of short interfering RNA (siRNA) knocking down RHO
[0104] Using short interfering RNA (siRNA) design principles described by Khvorova and colleagues 4,5 , 14 siRNAs were designed to specifically cleave dog siRNAs, 12 of which also targeted human rhodopsin mRNA. Prior to proceeding, positions 2 to 19 of siRNAs were screened against the NCBI Human RefSeq database using the NCBI Blast utility (blast.ncbi.nlm.nih.gov / Blast.cgi). Two potential siRNAs were excluded because they closely matched other genes likely to be expressed in the retina. RNA versions of the 10 siRNAs were ordered from GE Healthcare Dharmacon together with non-targeting siRNAs used as controls. These were expressed in conjunction with green fluorescent protein (GFP, Figure 11The exemplary plasmid profile depicted in ) was tested in cells fused to human RHO and the reduction in green fluorescent cells was measured by fluorescence activated cell sorting (FACS). The rationa...
Embodiment 2
[0111] Example 2: RHO + / + Analysis of RHO KD in dogs
[0112] Dog 2190 (rcd1 carrier) received subretinal injections of AAV2 / 5-sc-H1-shRNA-Rho131 at the concentrations listed in Table 6. Dogs were terminated 8 weeks after injection. Several 3 mm neuroretinal biopsy punches were collected from both the bleb and non-bleb areas of each eye.
[0113] Table 6: Dog 2190
[0114]
[0115] Some non-limiting examples of the AAV2 / 5-sc-H1-shRNA constructs described herein may also be referred to as AAV2 / 5-sc-MOP500rGFP-shRNA constructs. AAV2 / 5 means that the shRNA-encoding nucleic acid is flanked by the AAV2 ITRs and is provided in rAAV particles containing the AAV5 capsid protein. In some embodiments, any interfering RNA described herein and any recombinant RHO gene described herein can be present on the same AAV nucleic acid (e.g., flanked by AAV2 ITRs) in an rAAV particle (e.g., comprising an AAV5 capsid protein). ) available on . In some embodiments, any of the interfering...
Embodiment 3
[0121] Example 3: RHO + / + Further analysis of RHO KD in dogs
[0122] Dog 2194 (rcd1 carrier) received subretinal injections of AAV2 / 5-sc-H1-shRNA-Rho820 at the concentrations listed in Table 7. Dogs were terminated 8 weeks after injection. Several 3 mm neuroretinal biopsy punches were collected from both the bleb and non-bleb areas of each eye.
[0123] Table 7: Dog 2194
[0124]
[0125] Western blot analysis:
[0126] Two biopsy punches (representing bleb or non-bleb areas) from each eye were incubated in 50 [mu]l of Lewin's buffer solution A (containing protease inhibitors) for 15 minutes on ice. Samples were sonicated with 40% amplitude, 15secON / 10secOFF X8 pulses. Then, the samples were centrifuged and the pellet was discarded. The protein concentration in the supernatant was measured by the Bradford method. Immunoblot was performed on 1 μg of total protein to visualize rhodopsin (antibody used: Millipore MAB5356, in 1:1000 dilution in blocking buffer). An...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com