Compound lactobacillus plantarum preparation with blood sugar reducing function and application of preparation
A technology of Lactobacillus plantarum and compound bacterial agents, applied in the direction of Lactobacillus, medical preparations containing active ingredients, applications, etc., can solve problems such as gastrointestinal dysfunction, liver and kidney function impairment, and visual abnormalities, and achieve good results. Effects on blood sugar, lowering blood sugar, and increasing body weight
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1, the preparation of bacterial agent
[0030] 1. Preparation of bacterial agents
[0031] 1. Inoculate the test bacteria preserved in the glycerol tube into 10mL liquid MRS medium at an inoculum size of 2%, and culture it statically at 37°C for 16-18h.
[0032] 2. Inoculate the bacterial liquid obtained in step 1 into 10 mL of liquid MRS medium with an inoculum amount of 2%, and culture it statically at 37° C. for 16-18 hours.
[0033] 3. Inoculate the bacterial solution obtained in step 2 into 200 mL liquid MRS medium with an inoculum amount of 3%, and culture it statically at 37°C for 16-18 hours.
[0034] 4. Inoculate the bacterial solution obtained in step 3 into 5L liquid MRS culture medium with 3% inoculation amount, stir and ferment at 37°C and 120rpm for 16-18h (control pH6.5 during the fermentation process), then centrifuge at 4°C and 6000rpm for 10min , to collect the bacterial pellet.
[0035] 5. Take all the bacterial precipitates obtained in ...
Embodiment 2
[0048] Embodiment 2, the application of bacterial agent
[0049] 1. Preparation of bacterial solution
[0050] Dissolve every 1 g of the bacterium agent prepared in Example 2 in 0.85% sterile saline to obtain a diluted bacterium solution.
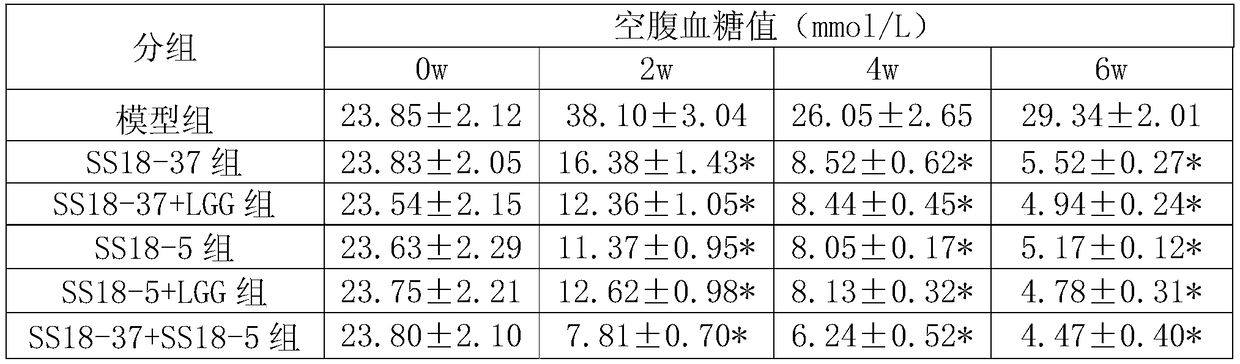
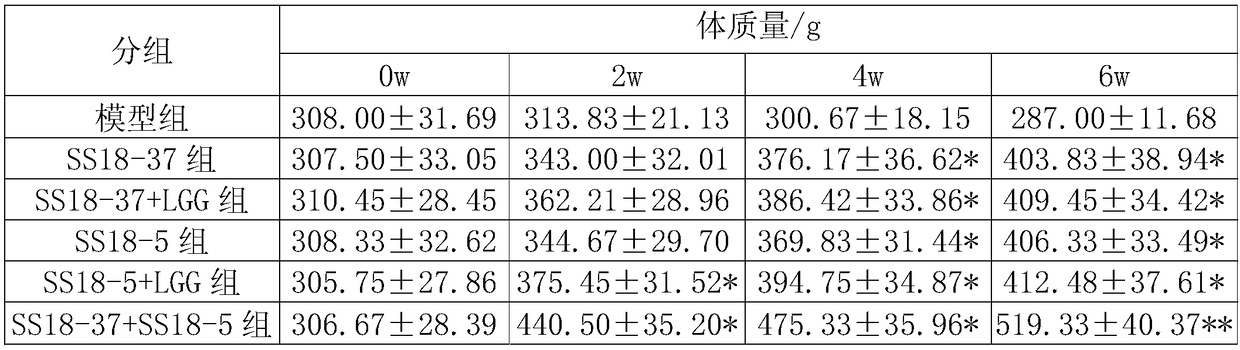
[0051] The SS18-37 bacterial agent prepared in Example 1 was used to obtain the SS18-37 bacterial liquid. In the SS18-37 bacterial liquid, the concentration of Lactobacillus plantarum SS18-37 was 1.0×10 9 CFU / mL.
[0052] The SS18-5 bacterial agent prepared in Example 1 was used to obtain the SS18-5 bacterial liquid. In the SS18-5 bacterial liquid, the concentration of Lactobacillus plantarum SS18-5 was 1.0×10 9 CFU / mL.
[0053] The SS18-37 / LGG composite bacterial agent prepared in Example 1 was used to obtain the SS18-37 / LGG bacterial liquid. In the SS18-37 / LGG bacterial solution, the concentration of Lactobacillus plantarum SS18-37 was 5.0×10 8 CFU / mL, the concentration of Lactobacillus rhamnosus LGG is 5.0×10 8 CFU / mL.
[0054] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com