A kind of preparation method and use of impurity compound in key intermediate for synthesizing sulpiride
A compound and impurity technology, applied in the field of medicine, can solve the problems of easy generation of impurities and unstable preparation process, and achieve the effect of ensuring drug safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Add 5.32g of compound II and 3.09g of compound III into the reaction flask, add 20ml of dichloromethane, after the dissolution is complete, add 1.50g of triethylamine, stir at 20°C for about 5h, the reaction is complete, wash twice with 50ml of purified water, reduce Concentrate under pressure to dryness to obtain an oily substance, add 30ml of purified water to precipitate a large amount of solids, filter the solids, rinse with 10ml of purified water, and dry in the air to obtain 4.32g of compound Ⅰ. Yield 80%, adopt HPLC peak area normalization method, record its content and be 97.175%
[0035] The chemical structural formula of compound I is:
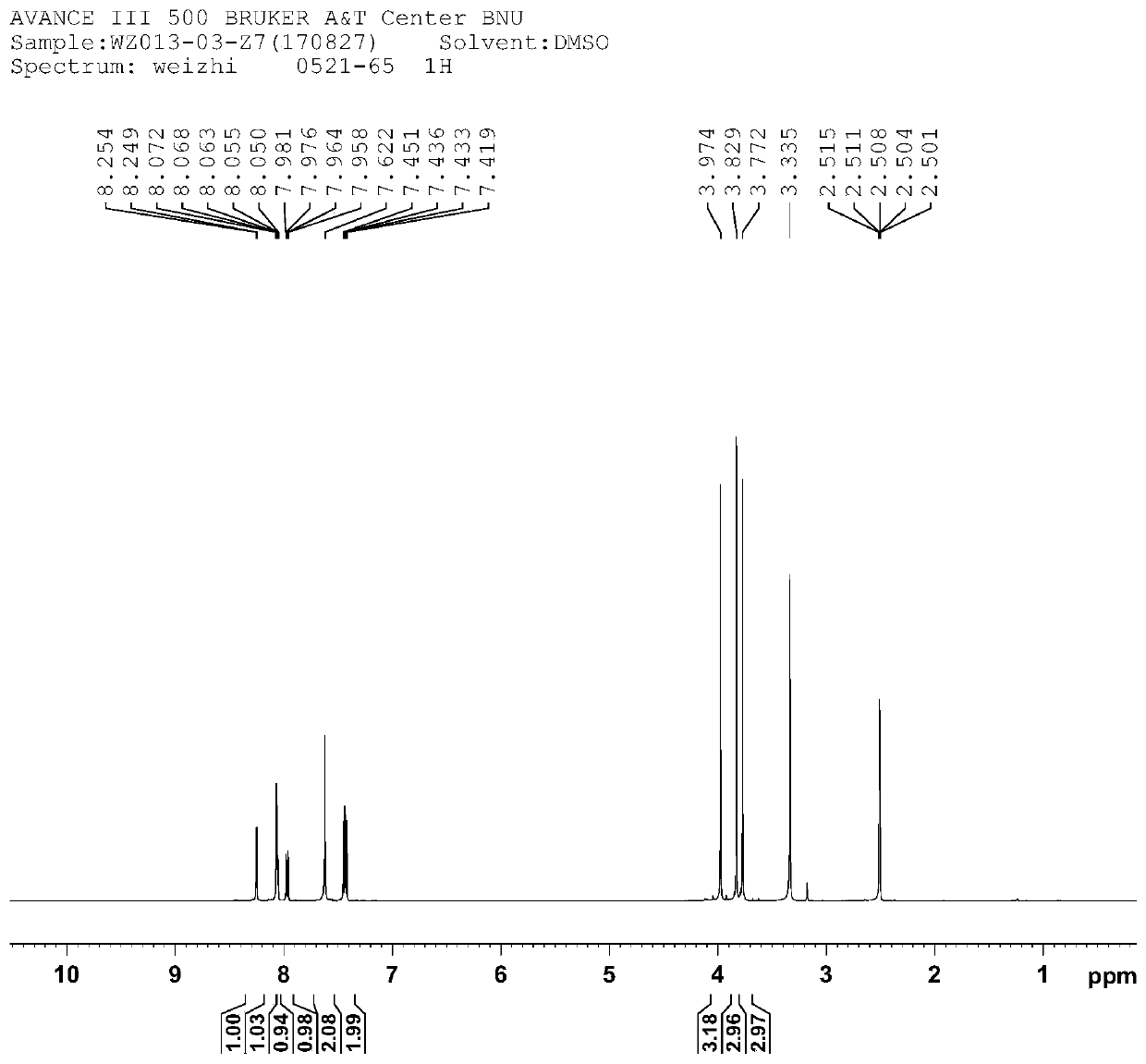
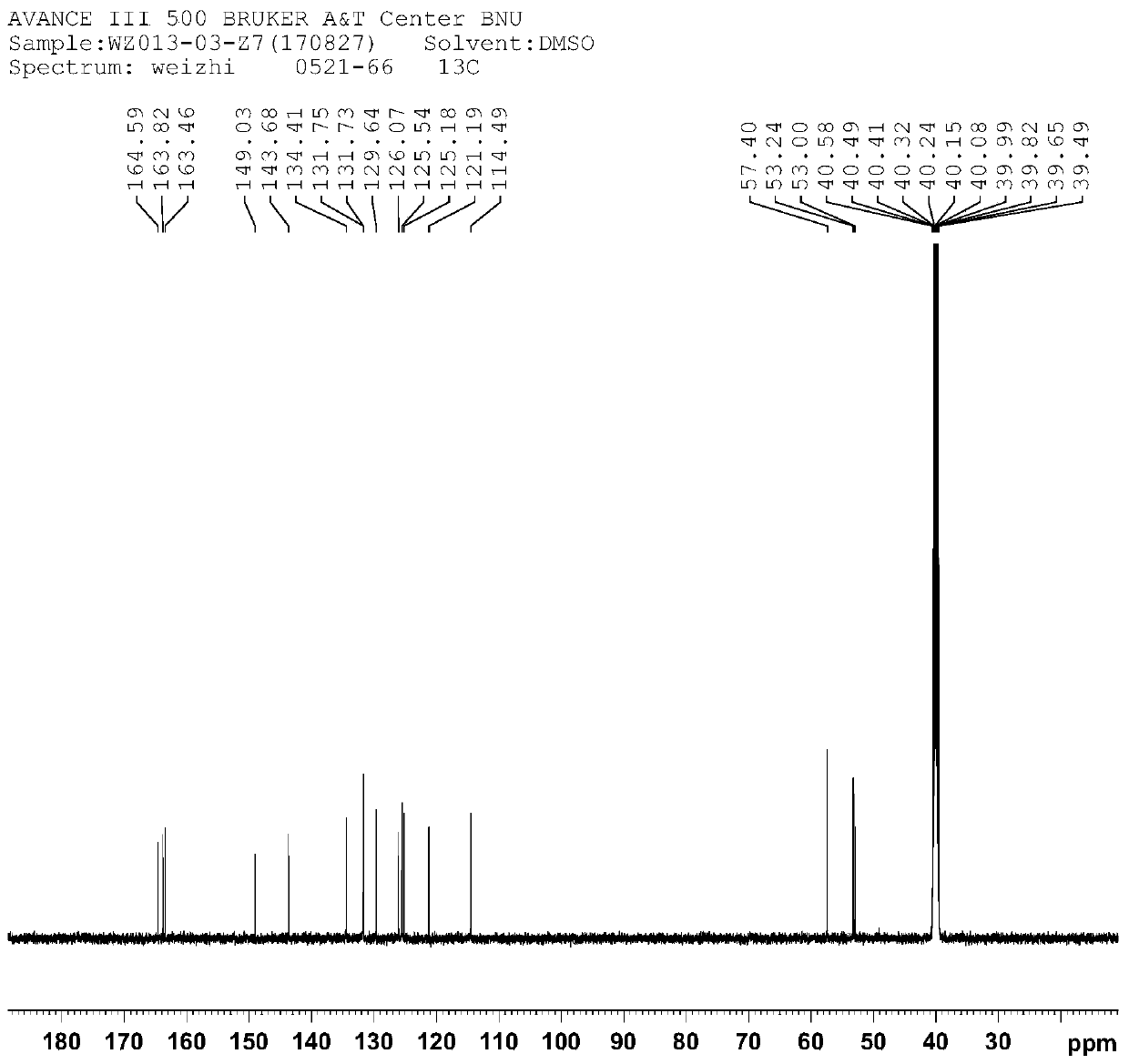
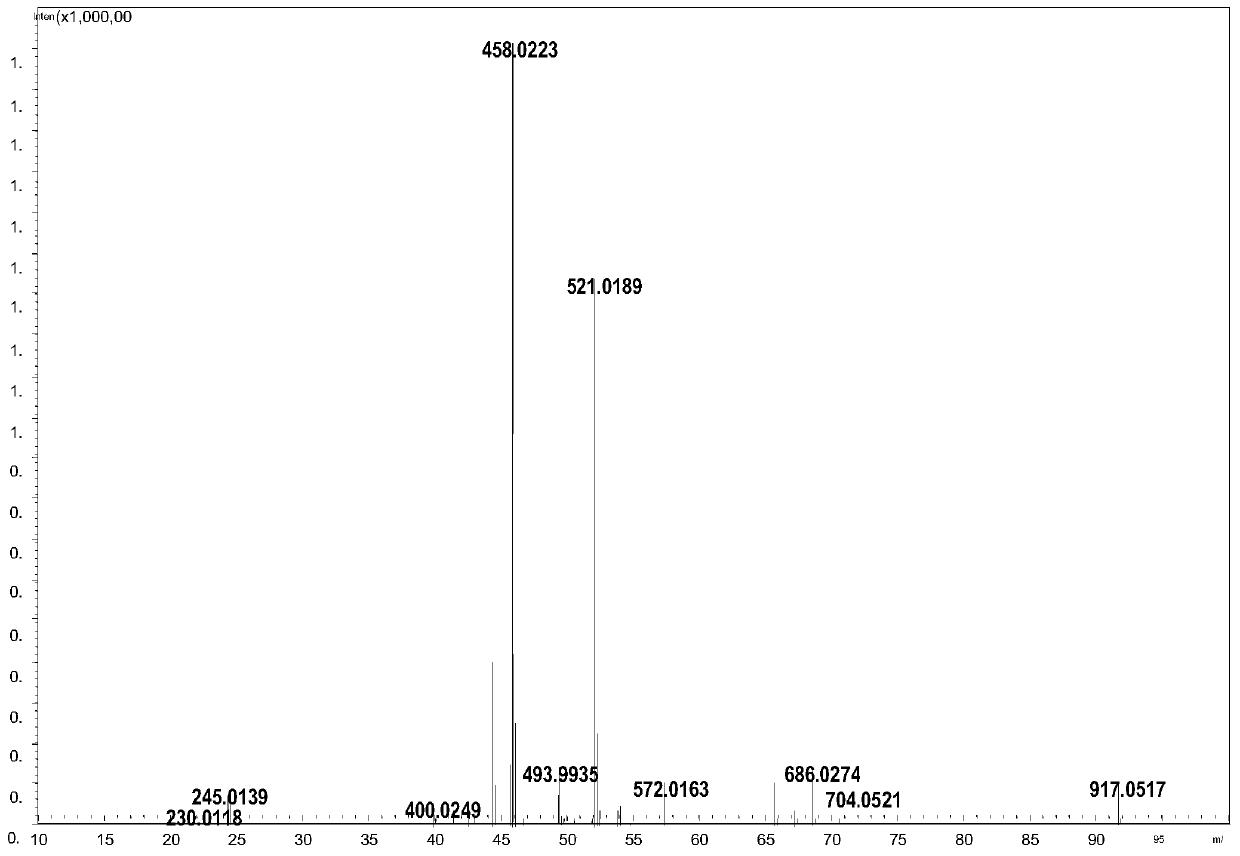
[0036] The structural characterization diagram of compound Ⅰ is shown in Figure 1-3 .
[0037] Structure analysis:
[0038]
[0039] from figure 1 It can be seen from the hydrogen spectrum that δ3.772ppm, δ3.829ppm, and δ3.974ppm are methyl hydrogen H-16 / H-8 / H-17; there are eight hydrogens in the benzene ring hydroge...
Embodiment 2
[0044] According to the synthetic method of sulpiride (Wang Fulan, Li Guiling, Tang Dengfeng. Synthesis of sulpiride, Chinese Journal of Pharmaceutical Industry, 1999, 27 (11): 487-488), compound IV was prepared, and 5.3 g of compound IV was used for preparative HPLC. Separation, the mobile phase used is acetonitrile-water (v:v=1:1), the detection wavelength is 240nm, the flow rate is 10ml / min, the column temperature is 30°C, the sample is injected into the chromatograph, the corresponding effluent is collected, and the product fractions are combined to receive the product The fractions were combined and concentrated to dryness under reduced pressure. The obtained compound I was 20mg, which was a white solid. The content was measured as 96.305% by HPLC peak area normalization method.
Embodiment 3
[0046] Application of compound Ⅰ as a reference substance in the impurity detection of sulpiride key intermediate 3 (compound Ⅳ)
[0047] Preparation of compound Ⅰ reference substance solution:
[0048] Take an appropriate amount of compound I prepared in the above example 1, put it in a 20ml volumetric flask, add acetonitrile aqueous solution, acetonitrile: water = 15:85 (v:v), dilute to the mark, ultrasonicate, shake well, and make each 1ml containing compound Ⅰ A solution of about 1 mg is used as the impurity control solution.
[0049] Preparation of the test solution:
[0050]Take an appropriate amount of compound IV sample prepared according to the above-mentioned synthetic method of sulpiride (Wang Fulan, Li Guiling, Tang Dengfeng. The synthesis of sulpiride, Chinese Journal of Pharmaceutical Industry, 1999, 27 (11): 487-488), put it in a 20ml volumetric flask, add Acetonitrile aqueous solution, acetonitrile: water=15:85 (v:v), dilute to the mark, ultrasonically dissol...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com