Improved preparations of adult liver progenitor cells
A technology of liver progenitor cells and cells, applied in the field of improved adult liver progenitor cell preparations, can solve the problem of impossible to determine liver progenitor cells and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0057] (b) a preparation of primary liver cells producing (a);
[0058] (c) culturing the cells contained in the preparation of (b) onto a support which allows the cells to adhere and grow thereon and the emergence of cell populations;
[0059] (d) passage the cells of (c) at least once; and
[0060] (e) Isolating the population of cells obtained after the passaging of (d) that is positive for the markers specified in the Summary of the Invention.
[0061] With respect to step (a) of the method, the dissociating step comprises obtaining an adult liver or a portion thereof containing an amount of primary cells useful for making HHALPCs together with fully differentiated hepatocytes. Liver primary cells are preferentially isolated from human liver tissue obtained from adult livers.
[0062] The term "liver" refers to the liver organ. The term "part of the liver" generally refers to a tissue sample derived from any part of the liver organ without any limitation on the amount o...
Embodiment 1
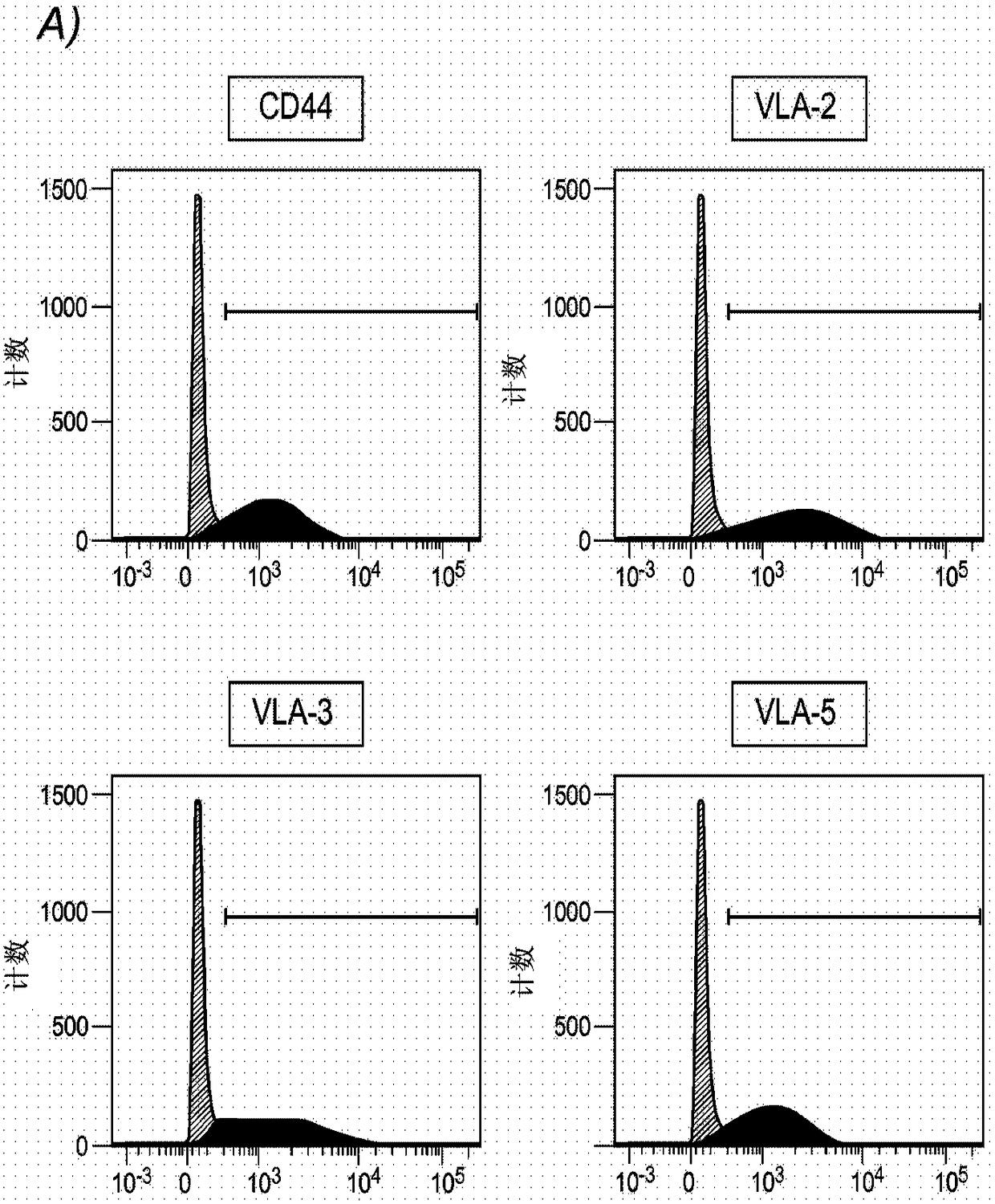
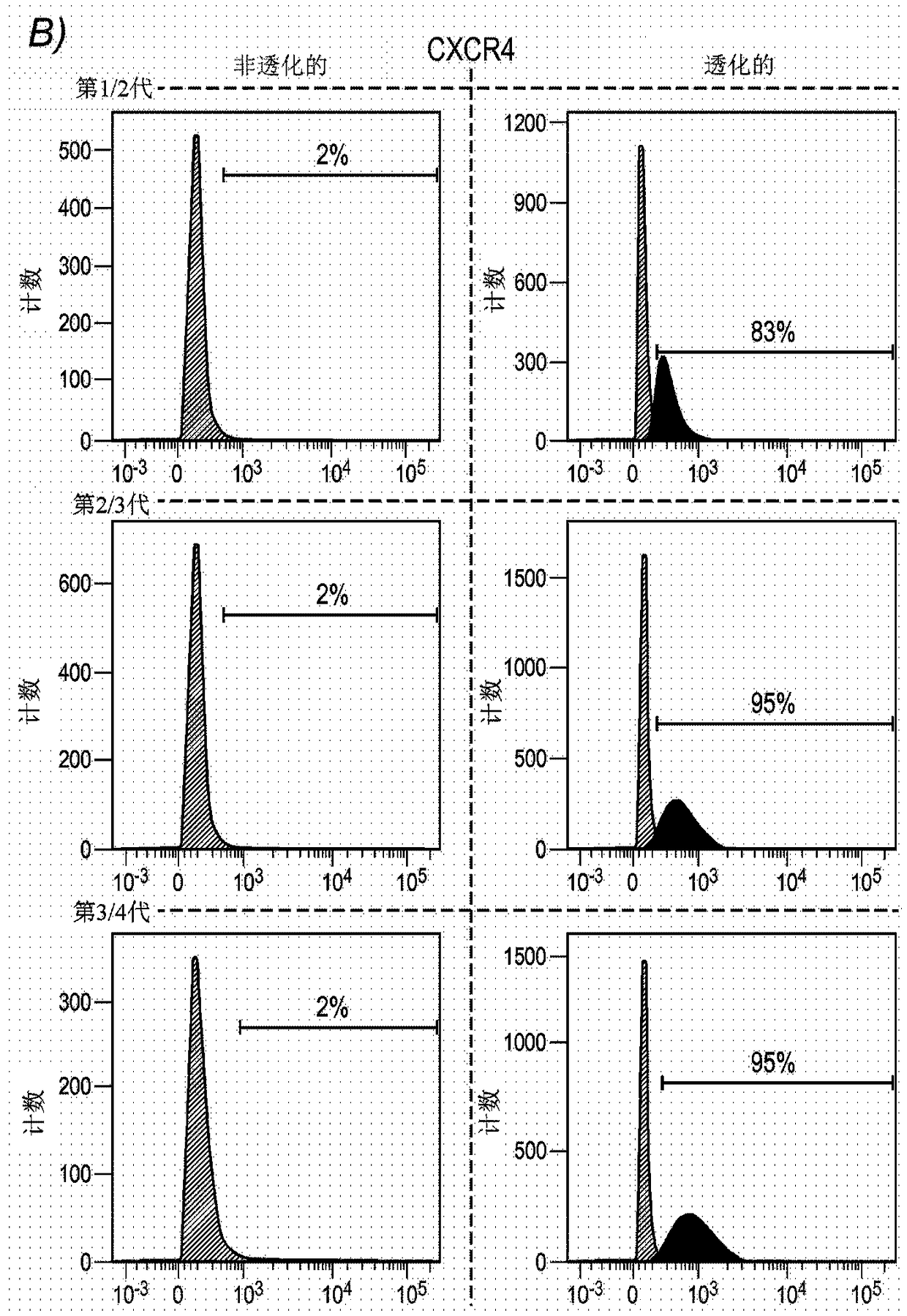
[0168] Example 1: Analysis of cell surface proteins on HHALPC
[0169] Materials and Methods
[0170] Isolation and Expansion of HHALPC in Cell Culture
[0171] HHALPC were recovered after primary culture of the hepatic parenchymal cell fraction was achieved after two steps of collagenase perfusion, filtration and low speed centrifugation using five different human donors as described elsewhere (Najimi M et al., 2007). These liver cells are suspended in cryopreservation medium prepared with ViaSpan solution and then maintained in liquid nitrogen by using appropriate vials, bags, or other systems for long-term storage and preservation of human cells. Cryopreserved hepatocyte suspensions were quickly thawed at 37°C and supplemented with 2.5g / L glucose, 0.084g / L bicarbonate and 5000IE / UI / ml heparin Wash twice with 10x volume of 5% human albumin. After centrifugation at 224 g for 10 min at 4 °C, the cell pellet was suspended in the desired cell culture medium.
[0172] HH...
Embodiment 2
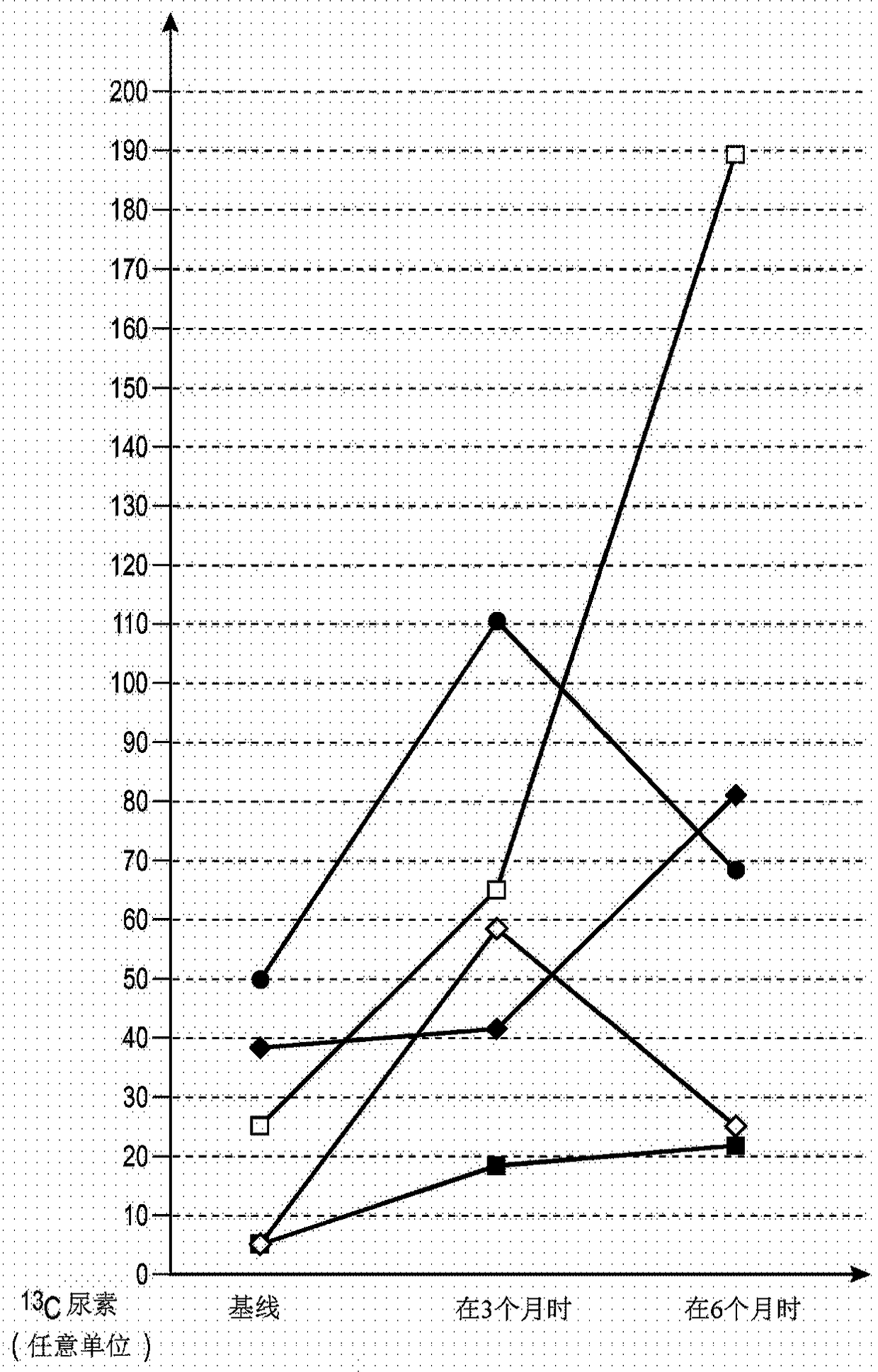
[0196] Example 2: Validation of the therapeutic properties of HHALPC
[0197] Materials and Methods
[0198] HHALPC preparation and administration to patients affected by urea cycle disorders
[0199] HHALPC were generated from healthy human liver cell suspension and expanded for 5 passages as shown in Example 1, then harvested, cryopreserved in CryoStor-10 (10% DMSO), and stored in liquid nitrogen. Before use, HHALPC were thawed and washed with albumin solution, then formulated in a 50ml plastic bag in a sterile environment in a GMP facility containing 250x10 in 0.084 sodium bicarbonate, 5% human albumin and 500IU heparin 6 cell suspension of cells. HHALPC was infused intravenously through a percutaneous transhepatic portal catheter at a flow rate of 0.5 to 2 ml / min, under general anesthesia, under radiological and ultrasound guidance, via direct transhepatic puncture of the right / left portal vein to the main artery at the confluence of the splenic and portal veins. Po...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com