A kind of gold plating solution and a kind of gold plating method
A gold-plating solution and electroplating solution technology, applied in the field of gold-plating solution and gold-plating, can solve problems such as the failure of industrialization of restrictive factors, and achieve the effect of avoiding fatal defects and clean production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0022] The gold plating solution provided by the present invention includes potassium iodoaurate at a concentration of 6-20 g / L, preferably 8-18 g / L, more preferably 10-15 g / L, calculated as Au. In the present invention, there is no special limitation on the source of the potassium iodaurate, and commercially available products well known to those skilled in the art or self-made potassium iodurate can be used. In the present invention, the preparation method of potassium iodaurate preferably comprises the following steps: (1) under stirring conditions, potassium iodide aqueous solution is added in batches in gold source aqueous solution, carry out precipitation reaction, after filtering, washing and Drying to obtain solid gold iodide; (2) adding the solid gold iodide obtained in the step (1) into potassium iodide aqueous solution, complexation reaction occurs, concentrated and crystallized, and dried to obtain potassium iodoaurate.
[0023] In the present invention, the molar ...
Embodiment 1
[0039] The gold plating solution is an aqueous solution comprising the following components: Potassium iodoaurate, calculated as Au, with a concentration of 20g / L, tetramethylammonium iodide 6g / L, EDTA 8g / L, sodium citrate 80g / L, citric acid 50g / L, cobalt sulfate heptahydrate 2g / L;
[0040] The preparation method of described Potassium Chloraurate comprises the following steps:
[0041](1) Under the condition of stirring at 300rpm, the potassium iodide aqueous solution that the concentration is 317g / L is added in batches to the chloroauric acid aqueous solution that the concentration is 100g / L, carries out precipitation reaction 30min, through filtering, washing and drying, obtains solid iodine Gold; Wherein, the molar ratio of potassium iodide and gold source is 3:1;
[0042] (2) the solid gold iodide that described step (1) is obtained is joined in the potassium iodide aqueous solution that concentration is 169g / L, complexation reaction 30min occurs, through concentrated c...
Embodiment 2
[0048] The gold-plating solution is an aqueous solution comprising the following concentrations of components: Potassium iodateaurate, calculated as Au, with a concentration of 6g / L, tetramethylammonium iodide 5g / L, EDTA 8g / L, sodium citrate 60g / L, citric acid 50g / L, nickel sulfate heptahydrate 2g / L; The preparation method of described potassium iodauric acid is identical with embodiment.
[0049] A gold plating method, comprising the steps of:
[0050] Place the copper substrate in the above gold plating solution for electroplating; the pH value of the electroplating solution is 4.5-5.2, the temperature of electroplating is 25°C, and the current density of electroplating is 0.4A / dm 2 , the area ratio of cathode and anode is 2:1; the electroplating time is 15s.
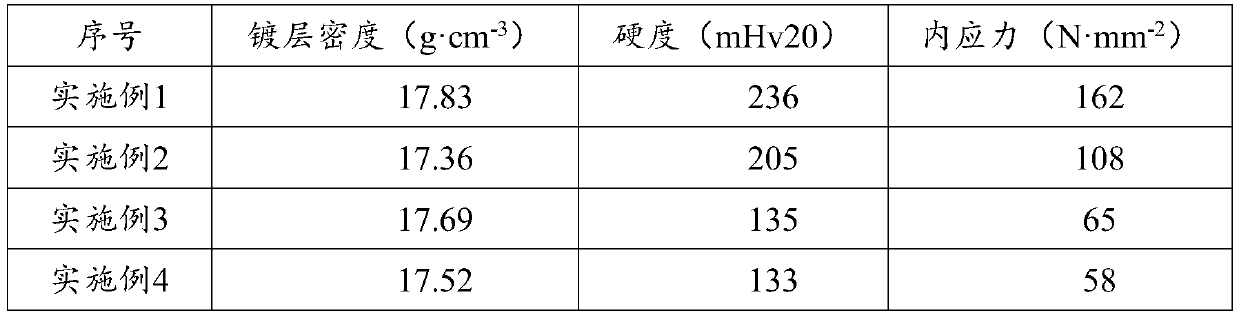
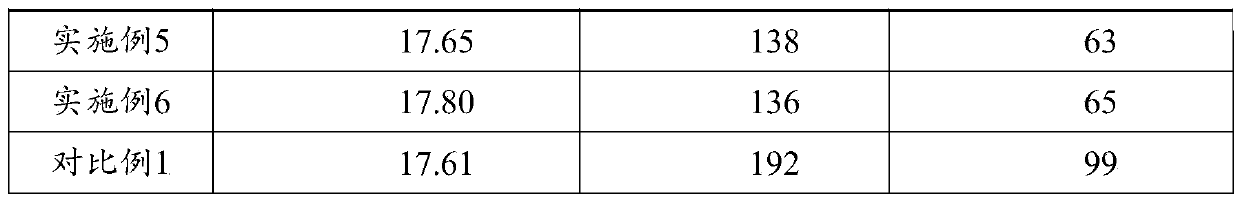
[0051] GB / T34625-2017 C-QA-LAB-006 was used to test the performance of the coating, and the results are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com