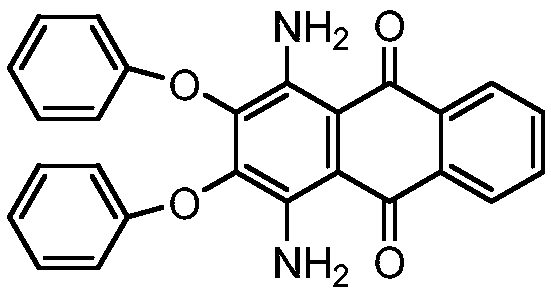
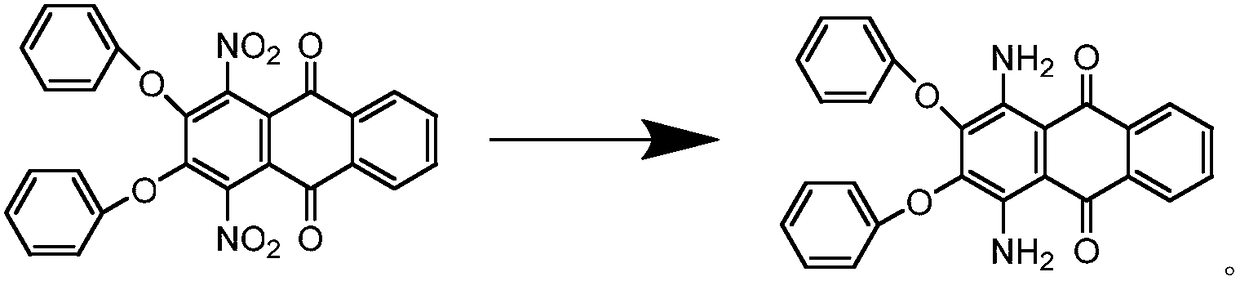
Synthesis process of solvent violet 59
A synthesis process, solvent violet technology, applied in the direction of amino-hydroxy anthraquinone dyes, anthracene dyes, organic dyes, etc., can solve the problems of low purity, low product yield, long reaction time, etc., to shorten the reaction time, yield The effect of high rate and reduction of insoluble matter
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
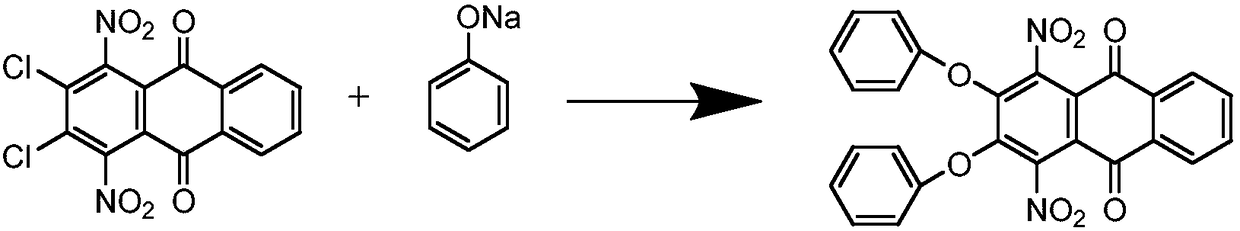
[0018] Step 1: Synthesis of 1,4-dinitro-2,3-diphenoxyanthraquinone
[0019] In a 500mL four-necked flask equipped with an electric stirrer, add 67.5g of sodium phenolate, 100g of 1,4-dinitro-2,3-dichloroanthraquinone and 185mL of o-dichlorobenzene, stir and heat up to 194℃, keep the temperature for reaction 5h. After the reaction, the temperature is lowered to 180°C, and the o-dichlorobenzene is desolvated. After the desolvation is completed, add 40ml NaOH (30%) and 100ml of water, stir at 100°C for 1h, filter, wash the filter cake with hot water, and dry . The product is 115.58 g, the yield is 88.2%, and the purity is 96.7%.
Embodiment 2
[0021] Other conditions are the same as in Example 1, and the experiment for detecting the mass ratio of different sodium phenolate / 1,4-dinitro-2,3-dichloroanthraquinone. The experimental results are shown in Table 1.
[0022] Table 1 Experiment of different mass ratios of sodium phenolate / 1,4-dinitro-2,3-dichloroanthraquinone
[0023]
[0024] From the above results, it can be seen that the mass ratio of sodium phenolate / 1,4-dinitro-2,3-dichloroanthraquinone is preferably 0.675 (Example 1).
Embodiment 3
[0026] Other conditions are the same as in Example 1, the experiment for detecting the mass ratio of different o-dichlorobenzene / 1,4-dinitro-2,3-dichloroanthraquinone, and the experimental results are shown in Table 2.
[0027] Table 2 Experiments on the mass ratio of different o-dichlorobenzene / 1,4-dinitro-2,3-dichloroanthraquinone
[0028]
[0029]
[0030] From the above results, it can be seen that the mass ratio of o-dichlorobenzene / 1,4-dinitro-2,3-dichloroanthraquinone is preferably 2.2 (Example 1).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com