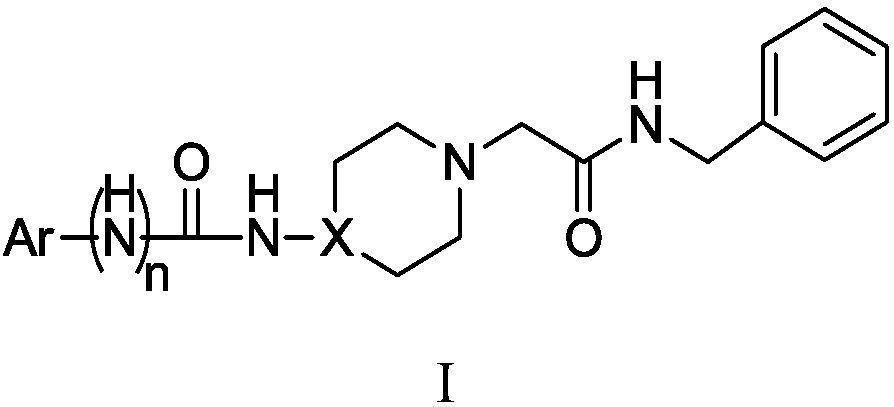
Acetylbenzylamine piperazine (piperidine) derivatives and application of derivatives as cerebral nerve protective agent
A technology of acetylbenzylamine piperazine and its derivatives, which is applied in the field of acetylbenzylamine piperazine derivatives, can solve the problems of imperfect clinical experiment schemes, toxic and side effects, narrow treatment time window, etc., and achieve high neuroprotective activity , small cardiac side effects, novel structure effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Example 1 Preparation of N-benzyl-2-(4-(3-phenylureido)piperidine)acetamide (T-1) and its salts
[0052] Using aniline as raw material, according to method 1, 0.51 g of the target product was obtained with a yield of 59.3%. ESI-MS[M+H] + : m / z=367.2, 1 H NMR (400MHz, DMSO-d6) δppm: 9.94 (s, 1H, CONH), 9.24-9.18 (m, 1H), 7.58 (s, 3H), 7.37-7.26 (m, 5H), 6.79 (d, J =4.0Hz,1H),6.88(t,J=8.0Hz,1H),4.37(d,J=8.0Hz,2H),4.05-3.98(m,2H),3.74-3.67(m,1H),3.49 (d,J=8.0Hz,2H),3.17(q,J=8.0Hz,1H),2.09-1.99(m,2H),1.91(s,1H),1.83-1.70(m,2H).
[0053] Preparation of compound T-1 hydrochloride
[0054] Compound T-1 (0.3g) and 5% hydrochloric acid aqueous solution (0.8mmol) were added to ethanol (10mL), refluxed and dissolved, and a white solid was precipitated by cooling, which was filtered to obtain 0.3g of white T-1 hydrochloride solid.
[0055] Preparation of compound T-1 mesylate
[0056] Compound T-1 (0.3g) and methanesulfonic acid aqueous solution (0.8mmol) were added to ethan...
Embodiment 2
[0061] Example 2 N-benzyl-2-(4-(3-(4-(trifluoromethyl)phenylureido)piperidine)acetamide (T-2) and its salt preparation
[0062] Using p-trifluoromethylaniline as raw material, according to method 1, 0.43 g of the target product was obtained with a yield of 55.4%. ESI-MS[M+H] + :m / z=435.2; 1 H NMR (400MHz, DMSO-d6) δppm: 9.83(s, 1H), 9.24-9.19(m, 1H), 8.92(s, 1H), 7.40-7.25(m, 7H), 7.05(t, J=8.0 Hz, 2H), 4.36(d, J=4.0Hz, 2H), 4.06-3.98(m, 2H), 3.72-3.65(m, 1H), 3.48(d, J=12.0Hz, 2H), 3.22-3.14 (m,1H),2.09-1.99(m,2H),1.91(s,1H),1.79-1.68(m,2H).
[0063] Preparation of compound T-2 hydrobromic acid salt
[0064] Using compound T-2 (2.0 mmol) and 5% hydrobromic acid aqueous solution (2.1 mmol) as raw materials, the preparation method of compound T-1 hydrobromide was used to obtain 0.9 g of white T-2 hydrobromide solid.
Embodiment 3
[0065] Example 3 Preparation of N-benzyl-2-(4-(3-(4-fluorophenyl)urea)piperidine)acetamide (T-3) and its salts
[0066] Using p-fluoroaniline as raw material, according to General Method 1, 0.87 g of the target product was obtained with a yield of 67%. ESI-MS[M+H] + :m / z=385.2; 1 H NMR (400MHz, DMSO-d6) δppm: 9.96(s, 1H), 9.12(t, J=8.0Hz, 2H), 9.24-9.18(m, 1H), 8.80(s, 1H), 7.38-7.25( m,7H),7.21(t,J=8.0Hz,2H),6.88(t,J=8.0Hz,1H),4.36(d,J=4.0Hz,2H),4.05-4.02(m,1H), 3.98(d, J=4.0Hz, 2H), 3.49(d, J=12.0Hz, 2H), 3.39(s, 1H), 3.18(q, J=12.0Hz, 1H), 2.04-1.99(m, 2H ),1.79-1.69(m,2H).
[0067] Preparation of Compound T-3 Fumarate
[0068] Using compound T-3 (2.3 mmol) and fumaric acid (2.4 mmol) as raw materials, the preparation method of compound T-1 hydrobromide was adopted to obtain 1.0 g of white solid.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Maximum tolerated dose | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com