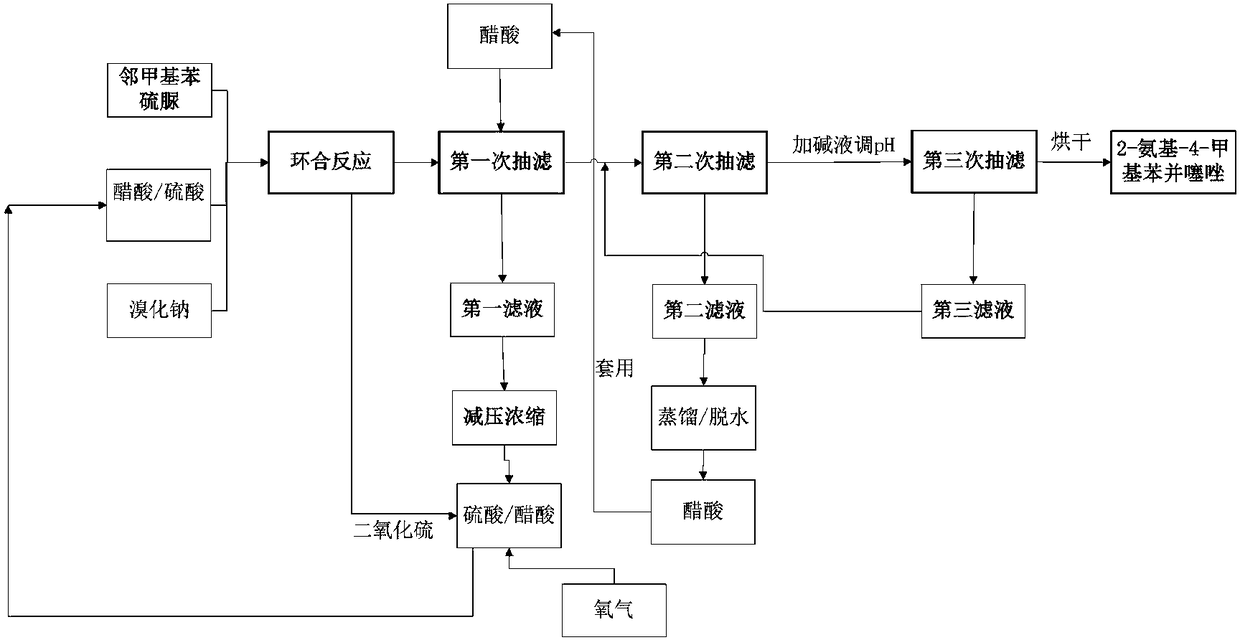
Preparation method of 2-amino-4-methylbenzothiazole
A technology of methylbenzothiazole and o-methylphenylthiourea, which is applied in the field of preparation of agricultural compounds, can solve the problems of narrow reaction temperature range, difficult treatment, and large alkali consumption, so as to reduce the difficulty and risk of operation and the amount of waste liquid The effect of reducing and reducing the amount of waste liquid
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Embodiment 1: Preparation of 2-amino-4-methylbenzothiazole
[0061] The implementation steps of this embodiment are as follows:
[0062] A. Ring closure reaction
[0063] According to the mass ratio of the total mass of acetic acid and sulfuric acid to o-methylphenylthiourea 4.5:1 and the mass ratio of acetic acid to sulfuric acid 0.8:1.0, add o-methylphenylthiourea and sulfuric acid to the acetic acid solvent, stir and mix evenly, and slowly heat to a temperature of 75°C, and at the same time send the escaped tail gas containing sulfur dioxide to the acetic acid sulfuric acid concentrate in step B, then add a sodium bromide catalyst according to the mass ratio of sodium bromide to o-methylphenylthiourea 0.03:1.0, and mix well , react at a temperature of 75° C. for 3 hours to obtain a 2-amino-4-methylbenzothiazole solution;
[0064] B. Filtration and washing
[0065] The temperature of the 2-amino-4-methylbenzothiazole solution obtained in step A is lowered to room t...
Embodiment 2
[0071] Embodiment 2: Preparation of 2-amino-4-methylbenzothiazole
[0072] The implementation steps of this embodiment are as follows:
[0073] A. Ring closure reaction
[0074] According to the mass ratio of the total mass of acetic acid and sulfuric acid to o-methylphenylthiourea 3:1 and the mass ratio of acetic acid to sulfuric acid 0.3:1.0, add o-methylphenylthiourea and sulfuric acid to the acetic acid solvent, stir and mix evenly, and slowly heat to a temperature of 84°C, and at the same time send the escaped tail gas containing sulfur dioxide to the concentrated solution of acetic acid and sulfuric acid in step B, then add a sodium bromide catalyst according to the mass ratio of sodium bromide to o-methylphenylthiourea 0.01:1.0, and mix well , react at a temperature of 95° C. for 1 h to obtain a 2-amino-4-methylbenzothiazole solution;
[0075] B. Filtration and washing
[0076] The temperature of the 2-amino-4-methylbenzothiazole solution obtained in step A is lowere...
Embodiment 3
[0082] Embodiment 3: Preparation of 2-amino-4-methylbenzothiazole
[0083] The implementation steps of this embodiment are as follows:
[0084] A. Ring closure reaction
[0085] According to the mass ratio of the total mass of acetic acid and sulfuric acid to o-methylphenylthiourea 5:1 and the mass ratio of acetic acid to sulfuric acid 1.0:1.0, add o-methylphenylthiourea and sulfuric acid to the acetic acid solvent, stir and mix evenly, and heat slowly to a temperature of 100°C, and at the same time send the escaped tail gas containing sulfur dioxide to the acetic acid sulfuric acid concentrate in step B, then add a sodium bromide catalyst according to the mass ratio of sodium bromide to o-methylphenylthiourea 0.06:1.0, and mix well , reacted at a temperature of 82°C for 2.4h to obtain a 2-amino-4-methylbenzothiazole solution;
[0086] B. Filtration and washing
[0087] The temperature of the 2-amino-4-methylbenzothiazole solution obtained in step A is lowered to room tempe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com