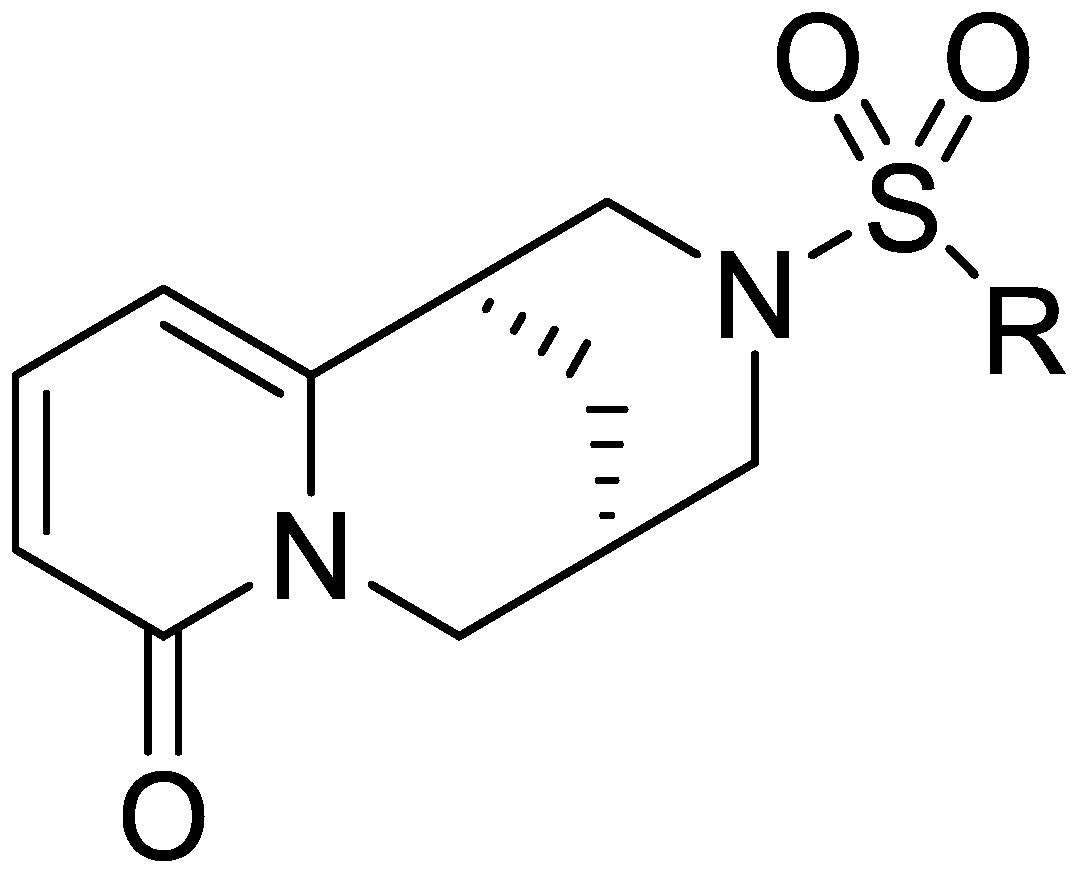
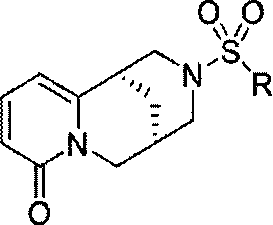
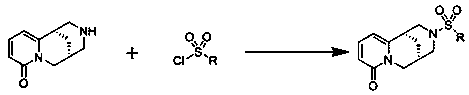Cytisine derivatives and their preparation and application in pesticides
A technology of cytisine and its derivatives, which is applied in the field of natural medicinal chemistry, can solve the problems of underived synthesis of cytisine, structure-activity relationship research, etc., and achieve high product purity, simple synthesis process, and good poisonous activity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Synthesis of N-2-naphthylsulfonyl-cytisine (1)
[0030] Its concrete synthetic operation is as follows:
[0031] With cytisine as reactant, triethylamine as catalyst, carry out sulfonylation reaction with 2-naphthalenesulfonyl chloride in organic solvent dichloromethane, wherein the moles of cytisine, 2-naphthalenesulfonyl chloride and catalyst The ratio is 5:10:15, and the amount of organic solvent added is 80 times the molar number of cytisine to obtain the cytisine derivative; Alkali derivative crude product, then the cytisine derivative crude product is separated by silica gel column chromatography with a volume ratio of 30:1:1 chloroform and methanol elution system to obtain high-purity N-2-naphthylsulfonyl - Cytisine.
[0032]
[0033] The detection data of the product are as follows: white solid, yield: 86%; 1 H NMR (400MHz, CDCl 3 )δ8.23(d,J=1.8Hz,1H),8.00–7.84(m,3H),7.71–7.58(m,2H),7.49(dd,J=8.6,1.9Hz,1H),7.15(dd ,J=9.1,6.7Hz,1H),6.36(d,J=9.1Hz,1H),5.94...
Embodiment 2
[0035] Synthesis of N-methylsulfonyl-cytisine (2)
[0036]Taking cytisine as reactant, taking triethylamine as catalyzer, carrying out sulfonylation reaction with methylsulfonyl chloride in organic solvent benzene, wherein the mol ratio of cytisine, methylsulfonyl chloride and catalyzer is 5: 10:20, the amount of organic solvent added is 85 times the molar number of cytisine, and cytisine derivatives can be obtained; after the sulfonylation reaction is completed, the crude product of cytisine derivatives is obtained after vacuum distillation , and then the crude product of cytisine derivatives is separated by silica gel column chromatography with the elution system of chloroform and methanol at a volume ratio of 30:2:1 to obtain high-purity N-methylsulfonyl-cytisine.
[0037]
[0038] The product detection data is as follows: white solid, yield: 88%; 1 H NMR (400MHz, CDCl 3 )δ7.41–7.25(m,1H),6.47(dd,J=9.1,1.4Hz,1H),6.07(dd,J=6.9,1.4Hz,1H),4.15(d,J=15.7Hz,1H ),3.98–3.82(m...
Embodiment 3
[0040] Synthesis of N-dimethylsulfonyl-cytisine (3)
[0041] With cytisine as reactant and triethylamine as catalyst, carry out sulfonylation reaction with dimethylaminosulfonyl chloride in organic solvent tetrahydrofuran, wherein the moles of cytisine, dimethylaminosulfonyl chloride and catalyst The ratio is 5:10:25, and the addition amount of organic solvent is 85 times of cytisine molar number, can obtain cytisine derivative; Base derivative crude product, then carry out silica gel column chromatography separation to the cytisine derivative crude product with the chloroform and methanol elution system of volume ratio 30:3:1 and can obtain high-purity N-dimethylsulfamoyl- Cytisine.
[0042]
[0043] The product detection data is as follows: white solid, yield: 89%; 1 H NMR (400MHz, CDCl 3 )δ7.37–7.21(m,1H),6.47(dd,J=9.1,1.4Hz,1H),6.08(dd,J=6.9,1.4Hz,1H),4.14(m,1H),3.90(m ,1H),3.70–3.60(m,1H),3.55(m,1H),3.19–3.04(m,3H),2.62–2.57(m,1H),2.52(s,6H),2.08–2.01(m ,1H),1.96–...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap