Method for constructing 2,4-diaryloxazole by acetophenone compound, ammonium persulfate and dimethylsulfoxide jointly
A technology of diaryloxazole and dimethyl sulfoxide, applied in directions such as organic chemistry, can solve the problems of low yield, high cost of raw materials, difficult to obtain, etc., and achieves simple operation, high raw material utilization rate, and pollution avoidance. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
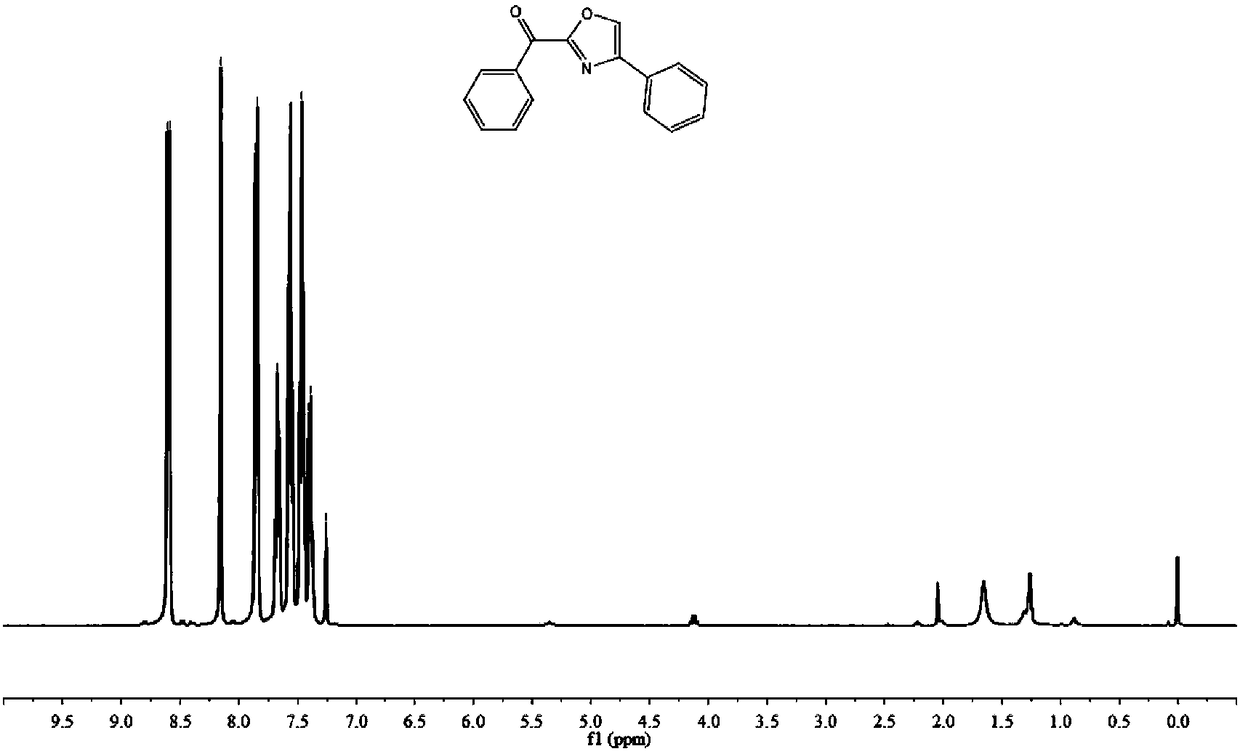
[0071]
[0072] 52.9 mg, 85% yield, dark yellow solid.
[0073] 1 H NMR (400MHz, CDCl 3 )δ8.60(d, J=7.7Hz, 2H), 8.15(s, 1H), 7.85(d, J=7.5Hz, 2H), 7.68(t, J=7.3Hz, 1H), 7.56(d, J=15.1Hz, 2H), 7.46(d, J=7.5Hz, 2H), 7.39(t, J=7.2Hz, 1H).
[0074] 13 C NMR (101MHz, CDCl 3 )δ178.71, 157.52, 142.68, 136.22, 134.94, 134.03,
[0075] 131.03, 129.91, 128.89, 128.86, 128.49, 125.83.
Embodiment 2
[0077]
[0078] 60.9 mg, 88% yield, orange solid.
[0079] 1 H NMR (400MHz, CDCl 3 )δ8.49(d, J=7.6Hz, 2H), 8.08(s, 1H), 7.72(d, J=7.1Hz, 2H), 7.34(d, J=7.8Hz, 2H), 7.25(d, J=5.3Hz,2H),2.45(s,3H),2.39(s,3H). 13 C NMR (101MHz, CDCl 3 )δ178.40, 157.59, 145.10, 142.64, 138.77, 135.67, 132.49, 131.16, 129.54, 129.22, 127.17, 125.73, 21.81, 21.32.
Embodiment 3
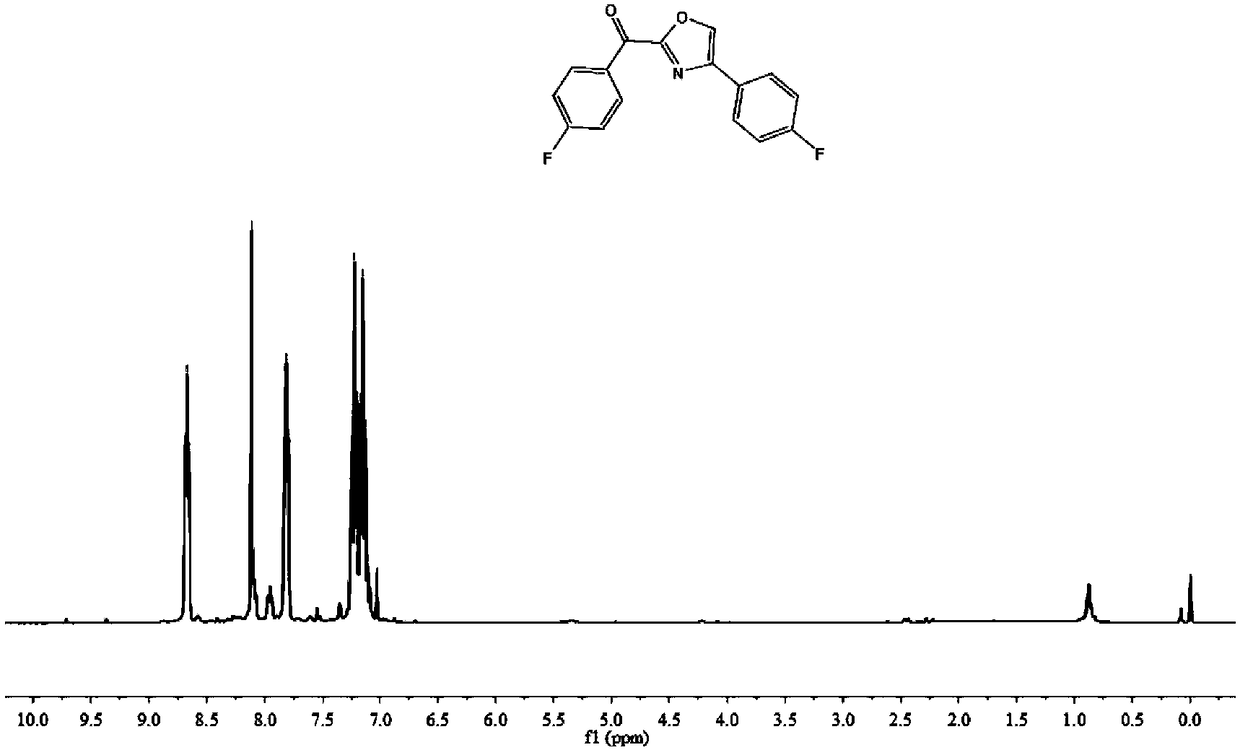
[0081]
[0082] 63.5 mg, 82% yield, yellow solid.
[0083] 1 H NMR (400MHz, CDCl 3 )δ8.64(d, J=7.7Hz, 2H), 8.04(s, 1H), 7.77(d, J=7.4Hz, 2H), 7.00(dd, J=17.3, 7.6Hz, 2H), 3.91( s,3H),3.85(s,3H).
[0084] 13 C NMR (101MHz, CDCl 3 )δ177.11, 164.37, 160.02, 157.67, 142.29, 134.90, 133.56, 127.97, 127.16, 122.70, 114.26, 113.80, 55.52, 55.32.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com