MiR-223-3p for inhibiting growth and transfer of osteosarcoma
A technology for osteosarcoma and osteosarcoma cells, which can be applied to antineoplastic drugs, medical preparations containing active ingredients, organic active ingredients, etc., and can solve the problems that miR-223-3p has not been studied in detail
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
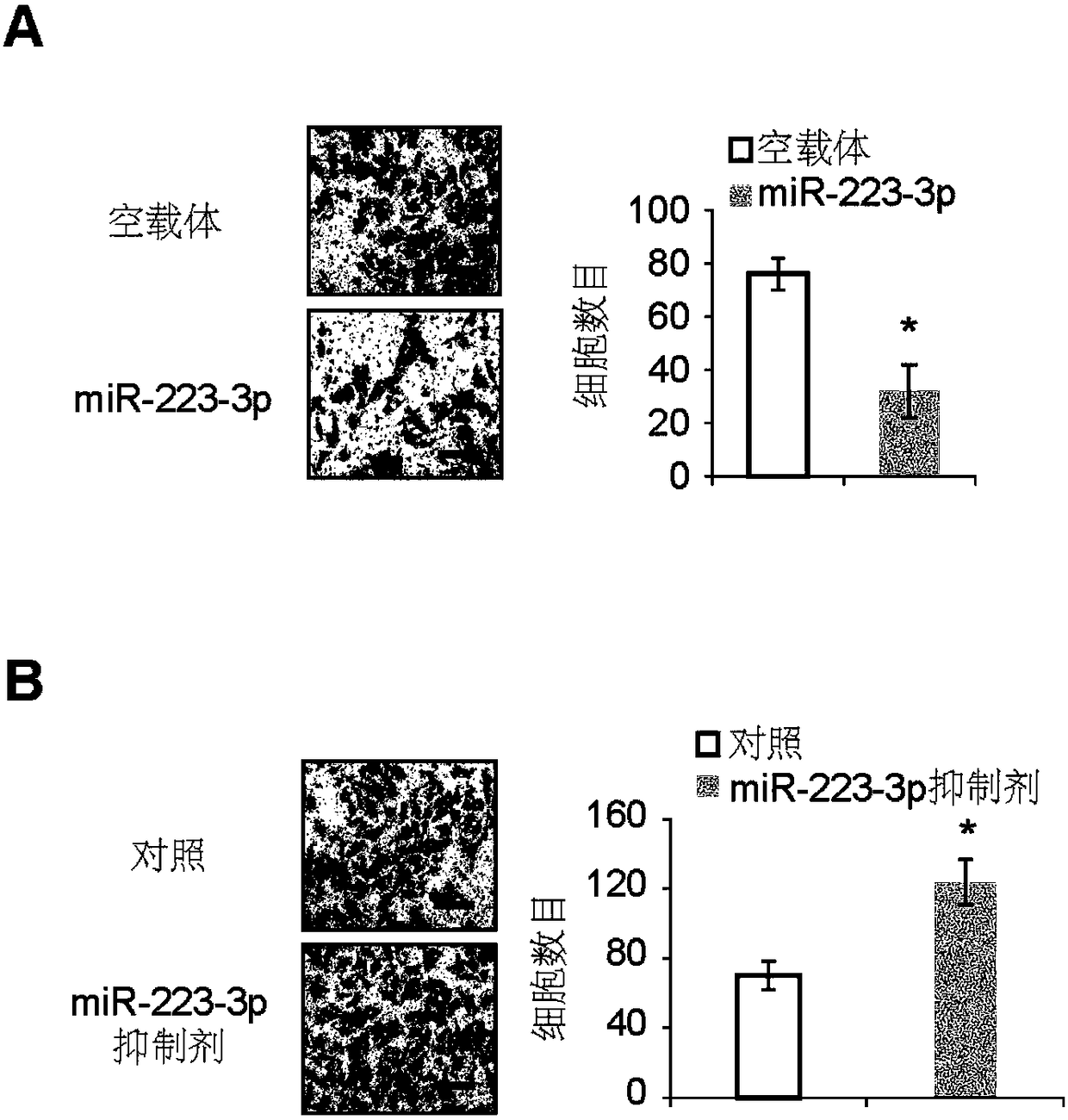
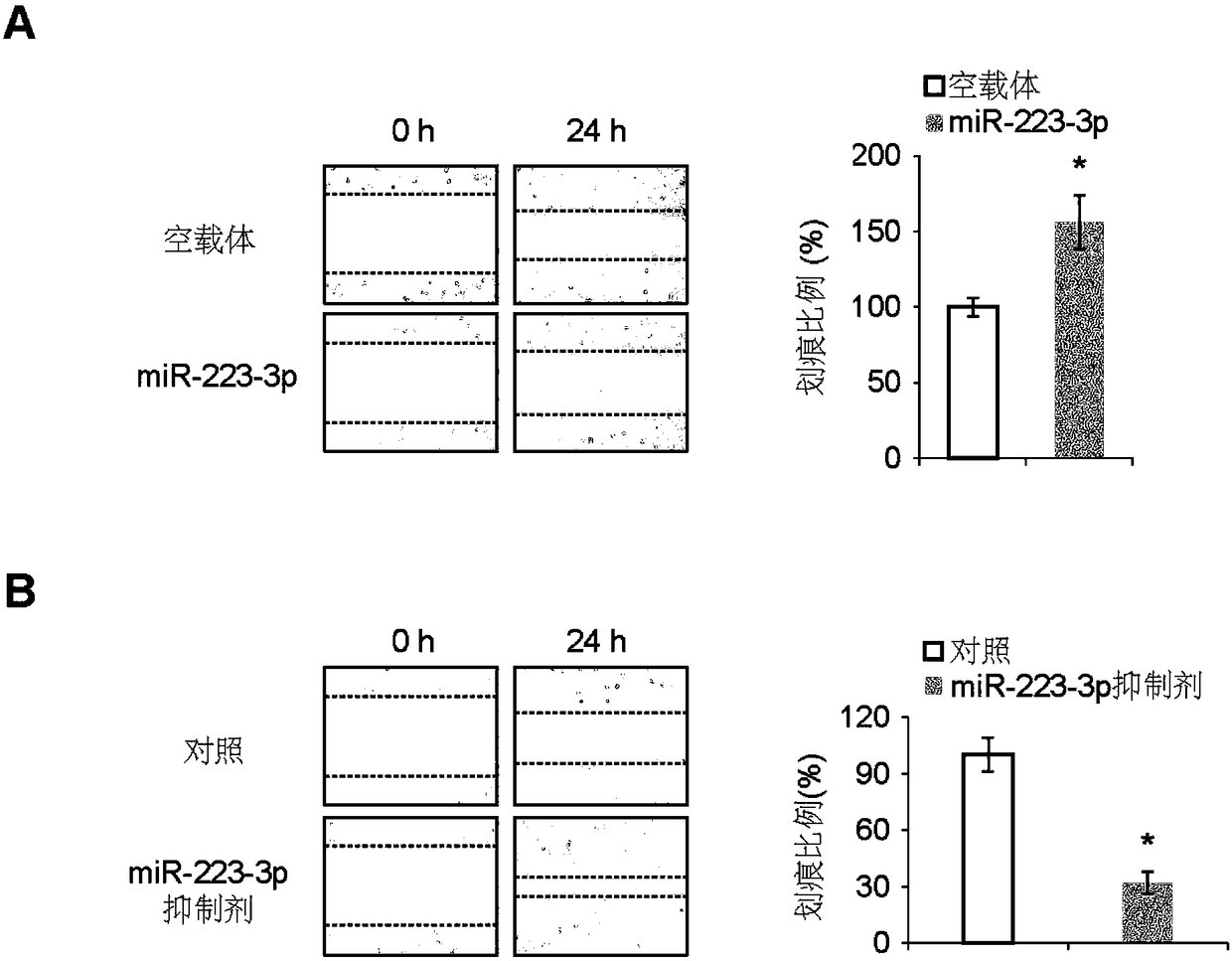
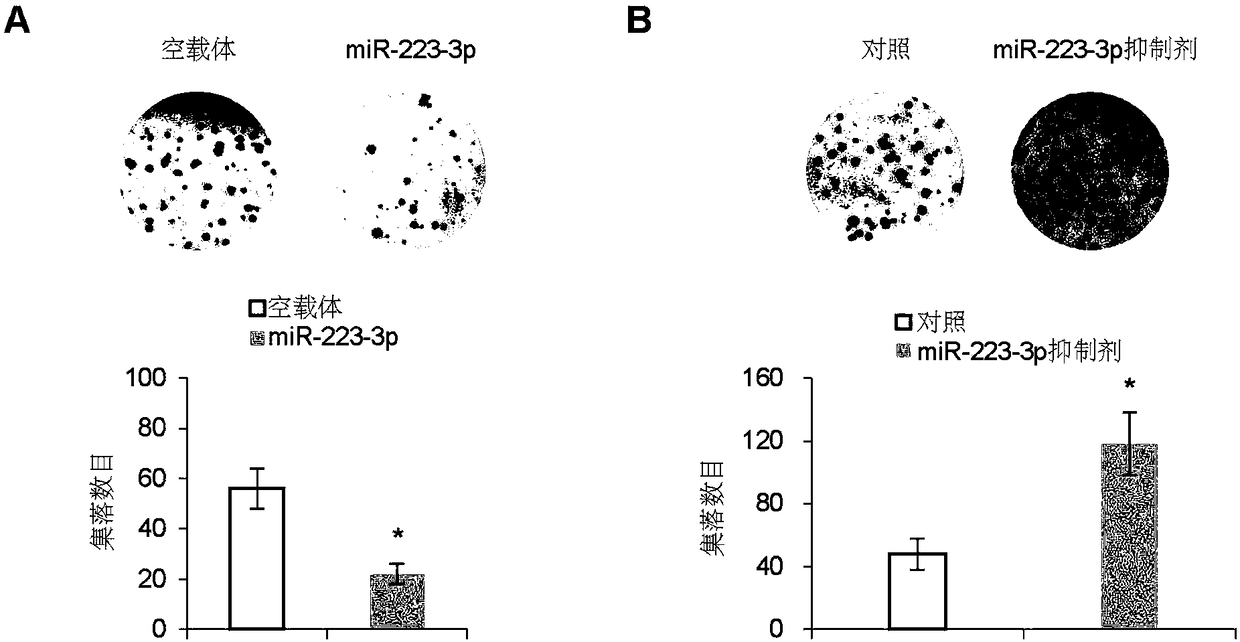
[0058] Example 1, miR-223-3p inhibits the growth and metastasis of osteosarcoma
[0059] 1. Preparation of miR-223-3p overexpressing osteosarcoma cell line
[0060] 1. Construction of miR-223-3p overexpression vector PCDH-miR-223-3p
[0061] According to the conventional PCR method, the encoding gene of miR-223-3p shown in the 501-610th positions of SEQ ID No.3 is used as a template, and the upstream primer and the downstream primer are used to carry out PCR amplification, wherein the upstream primer in the PCR primer: 5 '- GGATCC CCTGGCCTCCTGCAGTGCCA-3'; downstream primer: 5'- CTCGAG CTGGTAAGCATGTGCCGCACTT-3'. BamHI and XhoI double-digest the PCR product and the corresponding vector PCDH (product of System Biosciences, product number CD500B1), and insert the target fragment into the vector according to the correct phase. After transformation and screening, positive clones were identified by enzyme digestion, and the expression of various recombinant proteins was analyze...
Embodiment 2
[0080] Example 2, miR-223-3p is closely related to the clinical prognosis of patients with osteosarcoma
[0081] 1. Patients and Specimens
[0082] 133 cases of osteosarcoma and adjacent tissues were evaluated on the basis of the study of pathological and imaging criteria. Clinical information was obtained from patient visit records. Overall survival time was defined as the time from surgery to death. Patient follow-up data were updated monthly. Specimens were divided into two parts: one was immediately frozen in liquid nitrogen and stored at -80°C until RNA extraction, and the other was used for histopathological evaluation. Table 1 lists the clinical and demographic characteristics of the study population. This study was approved by the Institutional Review Board of the Chinese People's Liberation Army General Hospital (Beijing, China) and was performed with the informed consent of the patients.
[0083] Table 1 Correlation between miR-223-3p expression and clinicopatho...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com