Synthetic method of stannous chloride
A synthesis method, the technology of stannous chloride, applied in the direction of stannous chloride, tin halide, etc., can solve the problems of large amount of sewage, waste of resources, leakage of toxic and harmful gases such as chlorine gas, etc., and achieve the effect of reliable process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
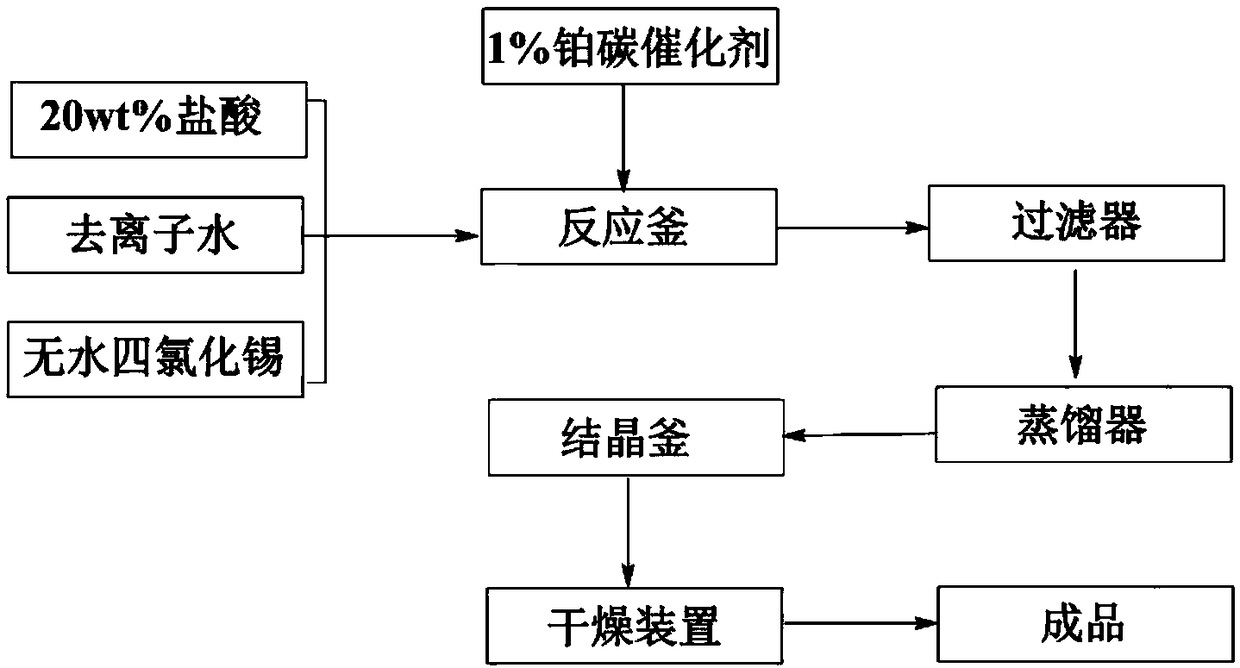
[0022] 1.97Kg of deionized water, 1.26Kg of 20wt.% hydrochloric acid, 0.77Kg of anhydrous tin tetrachloride and 50g of 1% ruthenium carbon catalyst were added to the reactor in sequence, the system was heated up to 110°C, 1.5MPa hydrogen gas was introduced, and 125°C Insulation reaction for 2 hours, after the reaction is completed, filter out the metal solid impurities; utilize the atmospheric pressure evaporation method to concentrate the formed tin protochloride solution until the temperature of the concentrated solution reaches 136 ° C, then pour out the concentrated solution and crystallize at room temperature; The obtained crystals were filtered under reduced pressure, and the filter cake was dried under vacuum at 80° C. with a vacuum degree of -0.05 MPa and a drying temperature of 50° C. for 12 hours to obtain white stannous chloride crystals with a content of 99.15%.
Embodiment 2
[0024] Add 1.97Kg of deionized water, 1.26Kg of 20wt.% hydrochloric acid, 0.77Kg of anhydrous tin tetrachloride and 50g of 1% rhodium carbon catalyst into the reactor in turn, raise the temperature of the system to 120°C, feed 3MPa hydrogen, and keep it warm at 130°C React for 6 hours, after the reaction is completed, filter out the metal solid impurities; utilize the atmospheric pressure evaporation method to concentrate the stannous chloride solution formed until the temperature of the concentrated solution reaches 136 ° C, then pour out the concentrated solution and crystallize at room temperature; The crystals were filtered under reduced pressure, the filter cake was dried at 80°C, the vacuum degree was -0.1MPa, the drying temperature was 90°C, and vacuum dried for 12 hours, and the white crystals of stannous chloride with a content of 99.33% were obtained.
Embodiment 3
[0026] Add 1.97Kg of deionized water, 1.26Kg of 20wt.% hydrochloric acid, 0.77Kg of anhydrous tin tetrachloride and 50g of 1% platinum-carbon catalyst into the reactor in sequence, raise the temperature of the system to 130°C, and feed 1.5MPa hydrogen gas into the reactor at 135°C Insulation reaction for 2 hours, after the reaction is completed, filter out the metal solid impurities; utilize the atmospheric pressure evaporation method to concentrate the formed tin protochloride solution until the temperature of the concentrated solution reaches 136 ° C, then pour out the concentrated solution and crystallize at room temperature; The obtained crystals were filtered under reduced pressure, and the filter cake was dried under vacuum at 80° C. with a vacuum degree of -0.05 MPa and a drying temperature of 50° C. for 12 hours to obtain white stannous chloride crystals with a content of 99.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com